BK101
Knowledge Base
Water - H2O
 Water or H2O, is a liquid necessary for the
life of most
animals and
plants.
A
binary compound that occurs at
room temperature as a clear colorless odorless
tasteless liquid; freezes into ice below zero degrees centigrade and
boils
above 100 degrees centigrade; widely used as a
solvent. The part of the
earth's surface covered with
water (such as a river or lake or
ocean). There are 3
atoms in a water
molecule, 2
hydrogen
atoms (H), and 1
oxygen Atom (O).
Water or H2O, is a liquid necessary for the
life of most
animals and
plants.
A
binary compound that occurs at
room temperature as a clear colorless odorless
tasteless liquid; freezes into ice below zero degrees centigrade and
boils
above 100 degrees centigrade; widely used as a
solvent. The part of the
earth's surface covered with
water (such as a river or lake or
ocean). There are 3
atoms in a water
molecule, 2
hydrogen
atoms (H), and 1
oxygen Atom (O). Over 70% of our
earth's surface is covered by water. 97.5% of all
water on earth is
salt water. Only 2.5% is fresh water,
and less then 1% is
drinkable.
70% of
fresh water is frozen in the
icecaps.
Water makes up to 60 percent of the
human body. Dehydration.
Over 70% of our
earth's surface is covered by water. 97.5% of all
water on earth is
salt water. Only 2.5% is fresh water,
and less then 1% is
drinkable.
70% of
fresh water is frozen in the
icecaps.
Water makes up to 60 percent of the
human body. Dehydration. Water Facts - Fluid Mechanics - Evaporation - Rain - Ice
Filters - Water Purification - Hydration
Testing - Monitoring - Quality of Water
Pesticides - Poisons - Water Pollution - Body Burden
2 Billion People Worldwide lack access to clean and safe drinking water.
Water Scarcity is the lack of fresh water resources to meet the standard water demand. Water scarcity can also be caused by droughts, lack of rainfall, over developed areas or pollution. In 2019 the World Economic Forum listed water scarcity as one of the largest global risks in terms of potential impact over the next decade. It is manifested by partial or no satisfaction of expressed demand, economic competition for water quantity or quality, disputes between users, irreversible depletion of groundwater, and negative impacts on the environment. Two-thirds of the global population (4 billion people) live under conditions of severe water scarcity at least 1 month of the year. Half a billion people in the world face severe water scarcity all year round. Half of the world's largest cities experience water scarcity. A mere 0.014% of all water on Earth is both fresh and easily accessible. Of the remaining water, 97% is saline and a little less than 3% is difficult to access.
The Average American uses 99 Gallons of Water a Day for activities like washing clothes, bathing, toilet-flushing and cooking. Then on top of that the average American uses another 250 gallons of water per day to generate daily electricity usage, = 350 gallons. Water Efficiency in Rural Areas is Getting Worse, Even as it Improves in Urban Centers.
Drought Monitor - Water Resources in the U.S.
Sectoral contributions to surface water stress in the coterminous United States.
News Deeply environmental, social and economic issues contributing to the drought crisis in California.
Climate Change - Global Change
Dry Land Farming - Irrigation - Hibernation
Desalination - Water from Air - Boiling Water
Public Water - Aquifers - Reservoirs - Wells
Water Use Tips - Conservation Methods - Run off
Since there are no federal regulations either guaranteeing a citizen’s right to water or water affordability, water and sewer prices more than doubled between 2000 to 2016, outpacing price increases for other basics such as electricity.
Clean Water Rights
Clean Water Act is the primary federal law in the United States governing water pollution. Its objective is to restore and maintain the chemical, physical, and biological integrity of the nation's waters by preventing point and nonpoint pollution sources, providing assistance to publicly owned treatment works for the improvement of wastewater treatment, and maintaining the integrity of wetlands.
Heavy Metals - Pesticides - Toxins - Dysentery
More than 2 billion people lack access to safe water, and more than 4.5 billion people lack adequate sanitation services.
Gov. Dannel P. Malloy Designated Connecticut’s Water Resources as a Public Trust - Friday, June 15, 2018.
Method to find toxic chemicals in drinking water. Chlorination generates hundreds of unregulated toxic byproducts in water. Among disinfection byproducts, only 11 compounds are currently regulated in drinking water, according to his paper published in the Royal Society of Chemistry journal Environmental Science.
Poison Papers. Documenting the Hidden History of Chemical and Pesticide Hazards in the United States.
Safe Drinking Water Act is the principal federal law in the United States intended to ensure safe drinking water for the public.
Over 1 Million American Rural Residents Don't Have Clean Water.
An Analysis of Water Collection Labor among Women and Children in 24 Sub-Saharan African Countries. An estimated 13.54 million women (and 3.36 million children) who are responsible for water collection trips that take 30 minutes or longer.
Lead in America's Water Systems is a National Problem. It has been four years since the story of lead-contaminated water in Flint, Michigan, first riveted the country. Meanwhile, a 2016 CNN report found that more than 5,000 U.S. water systems serving roughly 18 million people violated EPA rules for lead in water. 800 million children still exposed to lead.
Concentrations of antibiotics found in some of the world's rivers exceed 'safe' levels by up to 300 times, the first ever global study has discovered.
Community Water Center - Resource Conservation Recovery Act (wiki)
Superfund Act is a program designed to fund the cleanup of sites contaminated with hazardous substances and pollutants.
Emergency Planning and Community Right-to-Know Act to encourage and support emergency planning efforts at the state and local levels and to provide the public and local governments with information concerning potential chemical hazards present in their communities.
Drugs in Public Drinking Water
Nearly 200 million Americans across all 50 states are exposed to unsafe levels of chromium-6 or hexavalent chromium, a heavy metal known to cause cancer in animals and humans.
Michigan Mayor Declares State Of Emergency Over Lead Levels. (trail of disaster)
Mass Murderers are at it again. We have too many criminals in our local city governments. We need justice now!
HERE'S TO FLINT 2016 (youtube 44:54)
Financial Emergency in Michigan (wiki)
Receivership (wiki)
Elevated Levels of Lead in Children from Flint, Mich.
S.2377 - Lead-Free Drinking Water Act of 2004
CDC Lead
Additives may amplify the risk of pathogen release into drinking water. Many city drinking water systems add softening agents to keep plumbing free of pipe-clogging mineral buildup. According to new research, these additives may amplify the risk of pathogen release into drinking water by weakening the grip that bacteria -- like those responsible for Legionnaires' disease -- have on pipe interiors.
Today at least 4 million households have children living in them that are being exposed to high levels of lead. Almost 3,000 areas with poisoning rates far higher than in the tainted Michigan city. Yet many of these lead hotspots are receiving little attention or funding.
Diets High in Iron, calcium or vitamin C can limit the absorption of Lead in your Body and promote its excretion.
Our National Lead Problem Is Bigger Than Flint
America's lead poisoning problem isn't just in Flint. It’s everywhere. More than 5,000 water systems across the country are violating rules meant to keep lead out of drinking water, NRDC.
Nationally, nearly 1,400 water systems serving 3.7 million Americans violated the federal standard at least once over that time period. The information was based on data current as of September 2015. Corrosion Inhibitor (wiki)
In Connecticut 39 of 1,082 water systems serving schools, office parks, a state office, and apartment and condominium complexes have exceeded federal lead levels at least once since January 2013.
More than 6 million Americans are drinking water laced with unsafe levels of chemicals linked with cancer and other illnesses.
Prenatal immunotoxicant exposure and postnatal autoimmune disease.
Perfluorooctanesulfonic Acid
Indigenous Americans Have Been Living Flint's Nightmare for Decades
First Nations Water Crisis in Indigenous Communities
Deficits in Psychologic and Classroom Performance of Children with Elevated Dentine Lead Levels
What Do Parents Need to Know to Protect Their Children? Prevent lead exposure before it occurs.
$2.2 billion over five years to Service Men Poisoned by Water. Veterans, former reservists and former National Guard members who served for at least 30 days at the U.S. Marine Corps Base Camp Lejeune in North Carolina from 1953 to 1987, up to 900,000 service members, were potentially exposed to the tainted water at the base. They developed adult leukemia, aplastic anemia, bladder cancer, kidney cancer, liver cancer, multiple myeloma, non-Hodgkin’s lymphoma and Parkinson’s disease. Contaminants included the volatile organic compounds trichloroethylene, perchloroethylene, benzene and vinyl chloride.
Toxic Water Polluters List - Toxic Water - Polluters - Pollution of Groundwater
"No more Exemptions to the Law"
Groundwater Pollution can occur from on-site sanitation systems, landfills, effluent from wastewater treatment plants, leaking sewers, petrol stations or from over application of fertilizers in agriculture.
Toxic carcinogen found in 80 of New Jersey water systems
Legionnaires Disease comes from breathing in small water droplets or mist contaminated with the Legionella bacteria.
10 Biofilms in Drinking Water Distribution Systems: Significance and Control
Biofilm (wiki)
5.2 million Americans learned that their drinking water is contaminated with man-made Unsafe Levels of PFCs. DuPont, despite knowledge that the chemical was linked to increased rates of cancer and other horrific health conditions in animals and human beings, had dumped mountains of the stuff into the local water supply for decades. Mining.
Genx in drinking water in Raleigh, N.C. A chemical company under fire for releasing GenX into the Cape Fear River. GenX is a detergent used to make Teflon and other products it is related to a family of chemicals known to cause cancer and other Adverse health effects.
GenX is a chemical process that uses 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (FRD-903) and produces FRD-902 and E1. The process is proposed as a replacement for the use of Toxic and Carcinogenic PFOA (C8) for manufacturing fluoropolymers like teflon. Gen X was released by DuPont into the Cape Fear River which feeds the Wilmington, NC water supply for decades resulting in controversy over its adverse health effects. On November 2, 2017, a federal lawsuit was filed by the Brunswick County Government alleging that DuPont failed to disclose research regarding potential risks from the chemical.
3.4 million people die each year from water-related diseases. Water Facts.
Risk of infection from water in the air at home from the transmitting of bacterial disease via water spray from sinks, showers and toilets.
Body Burden (toxins that build up in the body)
Something in the water: Pollutant may be more hazardous than previously thought. Perchlorate, a chemical compound used in rocket fuels and other materials, may be a more hazardous pollutant than previously thought, says a new study. Sometimes toxins, such as hazardous wastes and industrial byproducts, seep into groundwater, the source of our drinking water.
Organizations that help Protect People and Clean Water Rights
Water for People - Agua 4 All
The Water Project - The Water Bearers
Water Integrity Network
Water Aid - World Water Day
The UNICEF Tap Project
Clean Water - Charity Water
Blue Planet Run - Safe Water
World Water Day (wiki)
Water.org - Water Credit
Water Standard - Water Smart Innovations
Natural Resources Defense Council helps defend our air, water, communities, and wild places and has worked to ensure the rights of all people to clean air, clean water, and healthy communities.
Earth Protectors - List of organizations that help protect the environment.
Sewage Bacteria Lurking in Hudson River Sediments. Study finds levels can greatly exceed those in water, with potential health risks. A new study shows that fecal bacteria from sewage are living in far greater quantities in near-shore sediments of the Hudson River than in the water itself. The river's pollution levels are generally monitored based on samples of clear water, not sediments, so the findings suggest that people stirring up the bottom while wading, swimming or kayaking may face previously unrecognized health risks.
Films about Water Pollution
Water Tariff is a price assigned to water supplied by a public utility through a piped network to its customers.
Cleveland's Water Rates more than Doubled – to $1,317 per year for an average family of four. And families in Detroit paid an astounding $1,151 annually. The average family pays $70 a month for water.
The Value of Water is raising awareness about the importance of water and the often invisible water challenges threatening our country.
Washington State takes bold step to restrict companies from Bottling Local Water. “Any use of water for the commercial production of bottled water is deemed to be detrimental to the public welfare and the public interest.” The move was hailed by water campaigners, who declared it a breakthrough.
Commodification of Water refers to the process of transforming water, especially freshwater, from a public good into a tradable commodity also known as an economic good. Sustainability.
Water Quality
Water Pollution is the contamination of water bodies (e.g. lakes, rivers, oceans, aquifers and groundwater). This form of environmental degradation occurs when pollutants are directly or indirectly discharged into water bodies without adequate treatment to remove harmful compounds. Water pollution affects the entire biosphere – plants and organisms living in these bodies of water. In almost all cases the effect is damaging not only to individual species and population, but also to the natural biological communities. Neutrons probe oxygen-generating enzyme for a greener approach to clean water.
Food and Water Watch
Water Quality refers to the chemical, physical, biological, and radiological characteristics of water. It is a measure of the condition of water relative to the requirements of one or more biotic species and or to any human need or purpose. It is most frequently used by reference to a set of standards against which compliance can be assessed. The most common standards used to assess water quality relate to health of ecosystems, safety of human contact, and drinking water. Drugs in water.
Drinking Water is water that is safe to drink or to use for food preparation, without risk of health problems. Globally, in 2015, 91% of people had access to water suitable for drinking. Nearly 4.2 billion had access to tap water while another 2.4 billion had access to wells or public taps. 1.8 billion people still use an unsafe drinking water source which may be contaminated by feces. This can result in infectious Diarrhea such as Cholera and typhoid among others. (Potable Water).
Water Filtering (clean water types)
Tap Water Quality Database Since 2010, water utilities' testing has found pollutants in Americans' tap water, according to an EWG drinking water quality analysis of 30 million state water records.
All 50 states in America have polluted waters where fish are unsafe to eat.
Bio-Assessment
Urban Aquaculture
City Fish Farmer
Urban Fish Farm (youtube)
River Protection
Water is Life (manual on amazon)
Recreational Water Quality Alerts
River Preservation Groups
Ocean Protection
Environmental Quotes and Sayings
Green Products
Fresh Water is naturally occurring water on Earth's surface in ice sheets, ice caps, glaciers, icebergs, bogs, ponds, lakes, rivers and streams, and underground as groundwater in aquifers and underground streams. Fresh water is generally characterized by having low concentrations of dissolved salts and other total dissolved solids.
How Nestle Makes Billions Bottling Free Water | Direct From With Dena Takruri - AJ+ (youtube) - Michigan’s water for next to nothing and sells it at great profit. And the state has just approved its request to pump even more, despite the failed promise of jobs and 80,000 public comments against Nestle.
Water Testing
Water Testing is a broad description for various procedures used to analyze water quality. Millions of water quality tests are carried out daily to fulfill regulatory requirements and to maintain safety.
Drinking Water Test Kit
Handheld TDS Meter
TDS-EZ Water Quality Tester
City Drinking Water Test Kits
Water Test Kits
Water Test America
Tox-Spot Water Toxicity Test
Special Pathogens Lab
Pesticides (Biomonitoring)
Water Canary
Low Cost inkjet Printed Nano
A pill with pesticide-detecting enzymes
Pullulan is used in various breath freshener or oral hygiene products.
Simple Paper-Strip Testing has the potential to tell us quickly what's in water, and other liquid samples from food, the environment and bodies -- Now researchers have developed a way to make these low-cost devices more versatile and reliable for analyzing both liquid and solid samples using adhesive tape. Paper Sensors
Paper-Based Device that cost one dollar and weighs a gram can be a simple way of testing water for contamination. The device consists of a microbial fuel cell (MFC), obtained by screen printing biodegradable carbon electrodes onto a single piece of paper. When these bacteria are exposed to polluted water, a change in the electric signal occurs, which can be used as a warning message that the water is unsafe to drink. An MFC is a device that uses the natural biological processes of 'electric' bacteria - attached to the carbon electrodes - to generate an electric signal and the sensor can be linked up with an electronic device such as a mobile phone, via a wireless transmitter, for a quick and user-friendly way of identifying if a water supply is safe to use.
Smartphone System to test for Lead in Water. Unlike most commercially available tests, it can detect levels below EPA standards.
Soil Testing
Smartphone-Based Paper Microfluidic Particulometry of Norovirus from Environmental Water Samples at the Single Copy Level. Human enteric viruses can be highly infectious and thus capable of causing disease upon ingestion of low doses ranging from 100to 102virions.Norovirus is a good example with a minimum infectious dose as low as a few tens of virions, that is, below femto-gram scale. Norovirus detection from commonly implicated environmental matrices (water and food) involves complicated concentration of viruses and/or amplification of the noro-virus genome, thus rendering detection approaches not feasible forfield applications. In this work, noro-virus detection was performed on a microfluidic paper analytic device without using any sample concentration or nucleic acid amplification steps by directly imaging and counting on-paper aggregation of antibody-conjugated, fluorescent submicron particles. An in-house developed smartphone-based fluorescence microscope and an image-processing algorithm isolated the particles aggregated by antibody-antigen binding, leading to an extremely low limit of norovirus detection, as low as 1 genome copy/µL in deionized water and 10 geno-mecopies/µL in reclaimed wastewater.
Water Filtering - Purification of Water
 Water Purification is the process of removing
undesirable chemicals, biological contaminants, suspended solids and
gases from water. The goal is to produce water fit for a specific purpose.
Most water is disinfected for human consumption (drinking
water), but water purification may also be designed for a variety of
other purposes, including fulfilling the requirements of medical,
pharmacological, chemical and industrial applications. The methods used
include physical processes such as filtration, sedimentation, and
distillation; biological processes such as slow sand filters or
biologically active carbon; chemical processes such as flocculation and
chlorination and the use of electromagnetic radiation such as ultraviolet
light. Purifying water may reduce the concentration of particulate matter
including suspended particles, parasites, bacteria, algae, viruses, fungi,
as well as reducing the concentration of a range of dissolved and
particulate matter.
Water Purification is the process of removing
undesirable chemicals, biological contaminants, suspended solids and
gases from water. The goal is to produce water fit for a specific purpose.
Most water is disinfected for human consumption (drinking
water), but water purification may also be designed for a variety of
other purposes, including fulfilling the requirements of medical,
pharmacological, chemical and industrial applications. The methods used
include physical processes such as filtration, sedimentation, and
distillation; biological processes such as slow sand filters or
biologically active carbon; chemical processes such as flocculation and
chlorination and the use of electromagnetic radiation such as ultraviolet
light. Purifying water may reduce the concentration of particulate matter
including suspended particles, parasites, bacteria, algae, viruses, fungi,
as well as reducing the concentration of a range of dissolved and
particulate matter.Water Filter removes impurities by lowering contamination of water using a fine physical barrier, a chemical process, or a biological process. Filters cleanse water to different extents for purposes such as providing agricultural irrigation, accessible drinking water, public and private aquaria, and the safe use of ponds and swimming pools. Information Filter.
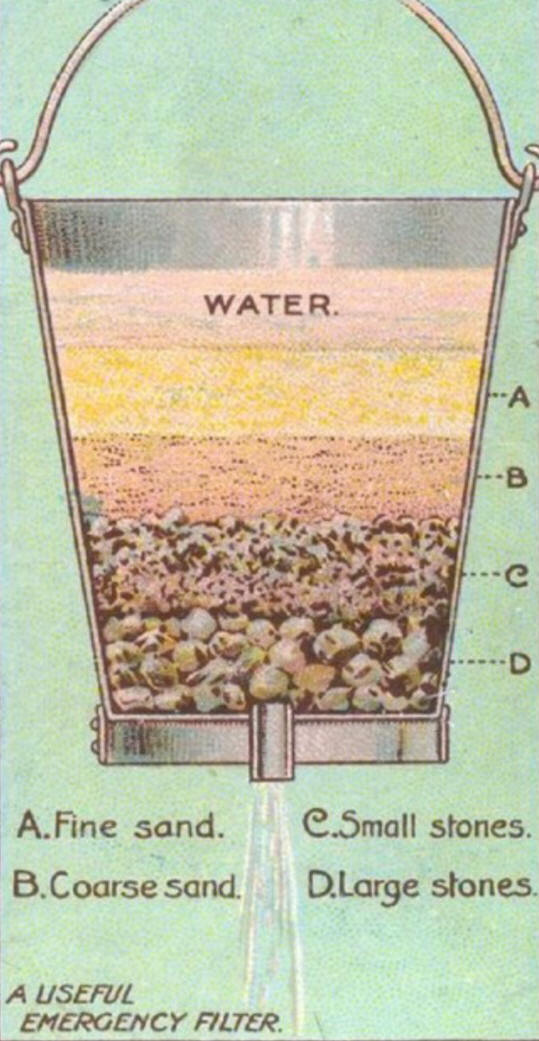
Media Filter is a type of filter that uses a bed of sand, peat, shredded tires, foam, crushed glass, geo-textile fabric, anthracite, crushed granite or other material to filter water for drinking, swimming pools, aquaculture, irrigation, stormwater management, oil & gas operations, and other applications. Desalination - Membranes.
Slow Sand Filter are used in water purification for treating raw water to produce a potable product. They are typically 1 to 2 metres deep, can be rectangular or cylindrical in cross section and are used primarily to treat surface water. The length and breadth of the tanks are determined by the flow rate desired by the filters, which typically have a loading rate of 200 to 400 litres per hour per square metre (or 0.2 to 0.4 cubic metres per square metre per hour).
Filtration is the process whereby fluids pass through a filter or a filtering medium that helps filter out impurities and toxins from the water to improve the water quality. There are basically 3 different types of filter medium: Mechanical, Biological and Chemical.
Biofilter is a pollution control technique using a bioreactor containing living material to capture and biologically degrade pollutants. Common uses include processing waste water, capturing harmful chemicals or silt from surface runoff, and microbiotic oxidation of contaminants in air.
Biosand Filter is a point-of-use water treatment system adapted from traditional slow sand filters. Biosand filters remove pathogens and suspended solids from water using biological and physical processes that take place in a sand column covered with a biofilm. BSFs have been shown to remove heavy metals, turbidity, bacteria, viruses and protozoa. BSFs also reduce discoloration, odor and unpleasant taste. Studies have shown a correlation between use of BSFs and a decrease in occurrence of diarrhea. Because of their effectiveness, ease of use, and lack of recurring costs, biosand filters are often considered appropriate technology in developing countries. It is estimated that over 200,000 BSFs are in use worldwide. Sand.
Biochar and Ultrasound Cleans Water. Scientists have developed a wastewater treatment process that uses a common agricultural byproduct to effectively remove pollutants and environmental hormones, which are known to be endocrine disruptors. Charcoal.
Activated Carbon is a form of carbon processed to have small, low-volume pores that increase the surface area available for adsorption or chemical reactions. Activated is sometimes substituted with active. Due to its high degree of microporosity, one gram of activated carbon has a surface area in excess of 3,000 m2 (32,000 sq ft) as determined by gas adsorption. An activation level sufficient for useful application may be obtained solely from high surface area. Further chemical treatment often enhances adsorption properties. Activated carbon is usually derived from charcoal. When derived from coal it is referred to as activated coal. Activated coke is derived from coke. Gravel - Pebbles.
Gravel and sand remove large and small particles, carbon removes pesticides, chlorine and other chemicals and improves the taste of water.
Researchers Remove Harmful Hormones from Las Vegas wastewater using Green Algae. A common species of freshwater green algae is capable of removing certain endocrine disrupting chemicals from wastewater, according to new research. Researchers explored the potential for use of a species of freshwater green algae called Nannochloris to remove EDCs from treated wastewater.
Portable Water Filters
Water Filter Kit and Faucet
Brita Water Filter Pitcher
Perfect Water Purifier
ZeroWater ZD-013 8-Cup Pitcher
Propur Water Filter Pitcher
Waves for Water Portable Filtration Systems.
Life Saver Water Bottle Filter Systems
Wateroam Fieldtrate Lite
LifeStraw Personal Water Filter
Katadyn Survivor 06 Desalinator
Katadyn Pocket an output of 1 liter/minute through our silver-impregnated ceramic filter.
Berkey Water Filters
SolarSack Water Purification is a special bag that is filled with four liters of water and placed in the sun for four hours. Using UVA and UVB rays, as well as heat from the sun, the water is cleaned of pathogenic bacteria. The user can then drink the water and reuse the bag for water purification.
Solar Pure Water - Desalination (salt water)
Removing Heavy Metals from Water in a matter of Seconds. Chemists have developed a new material that can remove heavy metals from water and make it drinkable in second.
Solar Still distills water, using the heat of the Sun to evaporate, cool then collect the water. There are many types of solar still, including large scale concentrated solar stills, and condensation traps (better known as moisture traps amongst survivalists). In a solar still, impure water is contained outside the collector, where it is evaporated by sunlight shining through clear plastic or glass. The pure water vapor condenses on the cool inside surface and drips down, where it is collected and removed. Distillation replicates the way nature makes rain. The sun's energy heats water to the point of evaporation. As the water evaporates, water vapor rises, condensing into water again as it cools and can then be collected. This process leaves behind impurities, such as salts and heavy metals, and eliminates microbiological organisms. The end result is pure distilled water.
The Solar Still Water Purification Kit (amazon)
Ultraviolet Water Purification is the most effective method for disinfecting bacteria from the water. Ultraviolet (UV) rays penetrate harmful pathogens in your home's water and destroy illness-causing microorganisms by attacking their genetic core (DNA).
UMD Researchers Work to Mitigate Water Scarcity Crisis with Solar-Powered Devices Made of Wood. Water can be transported through wood, purifying it for safe use.
To accurately taste water, water should be at room temperature, not chilled.
Amazon water Sanitation Hygiene Project
Moss Funaria Hygrometrica tolerates and absorbs an impressive amount of lead (Pb) from water which would be a green alternative for decontaminating polluted water and soil.
Quest Water Solutions
Dean Kamen (youtube)
Jerry Can Produces Potable Water
Reverse Osmosis is a water purification technology that uses a semipermeable membrane to remove ions, molecules, and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property, that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended species from water, including bacteria, and is used in both industrial processes and the production of potable water. 5 Stage Reverse Osmosis Water Filter (amazon).
Osmosis in biology and chemistry is the diffusion of molecules through a semipermeable membrane from a place of higher concentration to a place of lower concentration until the concentration on both sides is equal. Desalination.
Puralytics - Ozonated Water - Ozonator Purifier (amazon)
Water Ionizer is an home appliance which claims to raise the pH of drinking water by using electrolysis to separate the incoming water stream into acidic and alkaline components. Proponents claim that consumption of the alkaline stream results in a variety of health benefits, making it similar to the alternative health practice of alkaline diets. Such claims are not accepted in chemistry, physiology, and evidence-based medicine. Alkaviva - Ionizers - Make Alkaline Water (wiki-how).
Liquid Metal Discovery to make Toxic Water Safe and Drinkable. Researchers have discovered a revolutionary and cheap way to make filters that can turn water contaminated with heavy metals into safe drinking water in a matter of minutes. Nano-filters made of aluminium oxide could be cheaply produced using virtually no energy from a fixed amount of liquid metal Gallium. Gallium is a chemical element with symbol Ga and atomic number 31. It is in group 13 of the periodic table, and thus has similarities to the other metals of the group, aluminium, indium, and thallium. Gallium does not occur as a free element in nature, but as gallium(III) compounds in trace amounts in zinc ores and in bauxite. Elemental gallium is a soft, silvery blue metal at standard temperature and pressure, a brittle solid at low temperatures, and a liquid at temperatures greater than 29.76 °C (85.57 °F) (above room temperature, but below the normal human body temperature, (37.5 °C (99.5 °F)) hence, the metal will melt in a person's hands).The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. The alloy galinstan (70% gallium, 21.5% indium, and 10% tin) has an even lower melting point of −19 °C (−2 °F), well below the freezing point of water. Since its discovery in 1875, gallium has been used to make alloys with low melting points. It is also used in semiconductors as a dopant in semiconductor substrates. Gallium is predominantly used in electronics. Gallium arsenide, the primary chemical compound of gallium in electronics, is used in microwave circuits, high-speed switching circuits, and infrared circuits. Semiconducting gallium nitride and indium gallium nitride produce blue and violet light-emitting diodes (LEDs) and diode lasers. Gallium is also used in the production of artificial gadolinium gallium garnet for jewelry. Gallium has no known natural role in biology. Gallium(III) behaves in a similar manner to ferric salts in biological systems and has been used in some medical applications, including pharmaceuticals and radiopharmaceuticals.
Distilled Water is water that has been boiled into vapor and condensed back into liquid in a separate container. Impurities in the original water that do not boil below or near the boiling point of water remain in the original container. Thus, distilled water is one type of purified water. Distillation involves boiling the water and then condensing the steam into a clean container. Conductivity of Different Water Types.
You can drink distilled water but you might not like the taste because it's flatter and less flavorful than tap and bottled waters. Companies produce distilled water by boiling water and then condensing the collected steam back into a liquid. This process removes impurities and minerals from the water.
Vapor Distilled Water is a type of purified water that is created using a specialized heating process. It is freed of extra molecules and particulates, and is one of the "cleanest" forms of water that can be created in a lab. Vapor-distilled water does not occur naturally. Slingshot Water Vapor Distillation System.
PTG Wastewater Disinfection
Purified Water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Distilled water has been the most common form of purified water, but, in recent years, water is more frequently purified by other processes including capacitive deionization, reverse osmosis, carbon filtering, microfiltration, ultrafiltration, ultraviolet oxidation, or electrodeionization.
Ultrapure Water is water that has been purified to uncommonly stringent specifications. Ultrapure water is a commonly used term in the semiconductor industry to emphasize the fact that the water is treated to the highest levels of purity for all contaminant types, including: organic and inorganic compounds; dissolved and particulate matter; volatile and non-volatile, reactive and inert; hydrophilic and hydrophobic; and dissolved gases.
Capacitive Deionization s a technology to deionize water by applying an electrical potential difference over two electrodes, which are often made of porous carbon. Anions, ions with a negative charge, are removed from the water and are stored in the positively polarized electrode. Likewise, cations (positive charge) are stored in the cathode, which is the negatively polarized electrode. Today, CDI is mainly used for the desalination of brackish water, which is water with a low or moderate salt concentration (below 10 g/L). Other technologies for the deionization of water are, amongst others, distillation, reverse osmosis and electrodialysis. Compared to reverse osmosis and distillation, CDI is considered to be an energy-efficient technology for brackish water desalination. This is mainly because CDI removes the salt ions from the water, while the other technologies extract the water from the salt solution. Historically, CDI has been referred to as electrochemical demineralization, "electrosorb process for desalination of water", or electrosorption of salt ions. It also goes by the names of capacitive desalination, or in the commercial literature as "CapDI".
Water Revitalization Units work with natural energy - without the need for electricity or chemical additives. John Grander (wiki).
Hard Water - Soft Water
Aquaporin Water Purification
Aquaporin (wiki)
MSR SE200 Community Chlorine Maker
Zuvowater
Alter Ego Personal Water Filtration
Water Cones - Watercone® for Google Project 10^100 (youtube)
Portapure
Drinkpure Water Filtration Device
The Drinkable Book - Water is Life The Drinkable Book - Water is Life (youtube)
Janicki Omniprocessor (youtube)
Eureka Forbes AquaSure Amrit with Kitanu Magnet
Method of Binding Pollutants in Water
Arsenic Water Filter
Arsenic Removal Using Bottom Ash (ARUBA)
SE200™ Community Chlorine Maker
Zero Mass Water Hydropanel that makes drinking water from sunlight and air.
Low-Tech, Affordable Solutions to Improve Water Quality
System Selectively Sequesters Toxins from Water. Engineers develop technology to pull specific contaminants from drinking and wastewater, pipelines.
Filter water with a porous form of cyclodextrin polymer, superior to traditional activated carbon filters could be washed at room temperature with methanol or ethanol.
Novel Nanoparticle to remove Cadmium Toxicity from a Freshwater System.
Coffee-infused foam removes lead from contaminated water.
City Water Purification
Survival Kits (emergencies)
Electrospun Nanofibrous Membranes of Polyacrylonitrile/halloysite with Superior Water Filtration Ability. Electrospun polyacrylonitrile (PAN) nanofibrous membranes.
Water filtration breakthrough using metal-organic frameworks. Researchers discover efficient and sustainable way to filter salt and metal ions from water.
Metal-Organic Framework are compounds consisting of metal ions or clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. They are a subclass of coordination polymers, with the special feature that they are often porous. The organic ligands included are sometimes referred to as "struts", one example being 1,4-benzenedicarboxylic acid (BDC). More formally, a metal–organic framework is a coordination network with organic ligands containing potential voids. A coordination network is a coordination compound extending, through repeating coordination entities, in one dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating coordination entities in two or three dimensions; and finally a coordination polymer is a coordination compound with repeating coordination entities extending in one, two, or three dimensions. In some cases, the pores are stable during elimination of the guest molecules (often solvents) and could be refilled with other compounds. Because of this property, MOFs are of interest for the storage of gases such as hydrogen and carbon dioxide. Other possible applications of MOFs are in gas purification, in gas separation, in catalysis, as sensors and as supercapacitors. The synthesis and properties of MOFs constitute the primary focus of the discipline called reticular chemistry (from Latin reticulum, "small net"). Differently from MOFs covalent organic framework (COFs) are made entirely from light elements (H, B, C, N, and O) with extended structures. Matrix - Membranes
New material cleans and splits water. A photocatalytic system based on a material in the class of metal-organic frameworks. The system can be used to degrade pollutants present in water while simultaneously producing hydrogen that can be captured and used further. The first type of photocatalysis, hydrogen production, involves a reaction called “water-splitting”. The second type of photocatalysis is referred to as “organic pollutant degradation”, which refers to processes breaking down pollutants present in water.
Artificial Bio-Inspired Membranes for Water Filtration
Flower-like structure costs less than 2 cents and can produce more than half a gallon of water per hour per square meter. A technique that uses energy from sunlight to separate salt and other impurities from water through evaporation. Polypyrrole is a material known for its photothermal properties, meaning it's particularly good at converting solar light into thermal heat. The device collects water through its stem-like tube -- feeding it to the flower-shaped structure on top. It can also collect rain drops coming from above. Water finds its way to the petals where the polypyrrole material coating the flower turns the water into steam. Impurities naturally separate from water when condensed in this way. We designed the purification-collection unisystem to include a connection point for a low-pressure pump to help condense the water more effectively. The device removes any contamination from heavy metals and bacteria, and it removes salt from seawater, producing clean water that meets drinking standard requirements set by the World Health Organization. Our rational design and low-cost fabrication of 3D origami photothermal materials represents a first-of-its-kind portable low-pressure solar-steaming-collection system.
Water Taste: Water near the beach often has a slight scent of sulfur because of sulfur-producing microbes in groundwater. The stuff purified from some rivers or lakes can have an earthy, organic taste to it that results from leftover bits of decomposing plant matter. If you live in cities like New York or San Francisco, you enjoy pristine, delicious reservoir water piped in from distant mountains. Water bottled from mountain springs, like that from wells, can be packed with minerals that alter its flavor. Calcium makes water taste milky and smooth, magnesium can be bitter, and sodium makes it taste salty.
Water Sommelier
Adsorbent Materials for use in the industrial treatment of water have evaluated two types of phyllosilicates: a highly-charged expandable synthetic mica (Na-Mica-4), and one obtained from cation exchange with an organo-functionalised mica (C18-Mica-4). Phyllosilicates are a subclass of silicates and include common mineral in very different environments. The results show that the material C18-Mica-4 is capable of eliminating the majority of pollutants that were evaluated in urban waste water, as well as surface water and potable water. The study, also, provided data on the adsorption mechanism and establishes a significant correlation between the physical-chemical properties of the selected criteria and emerging pollutants and the adsorption to the material. In total, 18 organic pollutants were studied, among which were industrial pollutants, personal care products, and the pharmacologically active ingredients such as anti-inflammatories, antibiotics, anti-epileptics, central nervous system stimulants and lipid-lowering agents, among others. Nine active pharmacological ingredients were also tested (diclofenac, ibuprofen, salicylic acid, trimethoprim, carbamazepine, propranolol, caffeine, clofibric acid and gemfibrozil). Taken to achieve different therapeutic effects, these all end up polluting our waters, essentially, via human excretion. The study was carried out on untreated urban wastewater, treated urban wastewater, surface water from rivers and potable water.
Adsorb is to accumulate liquids or gases on the surface. Water Soluble (vitamins)
Adsorbent is a material having capacity or tendency to adsorb another substance.
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent), respectively. Adsorption is a surface-based process, while absorption involves the whole volume of the material. The term sorption encompasses both processes, while desorption is the reverse of it. Adsorption is a surface phenomenon.
Sponge is a multicellular organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like mesohyl sandwiched between two thin layers of cells. The branch of zoology that studies sponges is known as spongiology.
New process could safeguard water quality, environment and health. Pioneering single process can remove pollutants from waste water.
New System Recovers Fresh Water from Power Plants. About 39 percent of all the fresh water withdrawn from rivers, lakes, and reservoirs in the U.S. is earmarked for the cooling needs of electric power plants that use fossil fuels or nuclear power, and much of that water ends up floating away in clouds of vapor. But the new MIT system could potentially save a substantial fraction of that lost water -- and could even become a significant source of clean, safe drinking water for coastal cities where seawater is used to cool local power plants. When air that's rich in fog is zapped with a beam of electrically charged particles, known as ions, water droplets become electrically charged and thus can be drawn toward a mesh of wires, similar to a window screen, placed in their path. The droplets then collect on that mesh, drain down into a collecting pan, and can be reused in the power plant or sent to a city's water supply system.
Purifying water with the help of wood, bacteria and the sun. According to the United Nations, about one-fifth of the world's population lives in areas where water is scarce. Therefore, technologies to produce clean water from undrinkable sources, such as seawater, river or lake water, and contaminated water, are urgently needed. Now, researchers have developed a wood-based steam generator that, with the help of bacterial-produced nanomaterials, harnesses solar energy to purify water.
Desalination - Clean Drinking Water from Seawater
Desalination is the removal of Salts and Minerals from sea water to produce water suitable for human consumption or irrigation. The most commonly used technology for desalination is reverse osmosis (RO), a process in which seawater is forced through a membrane capable of removing salts and other small molecule contaminants. While the use of RO continues to rise around the world, many of its drawbacks, which include high energy consumption and a propensity for membranes to foul, continue to plague the industry. How Seawater Desalination Works (youtube)
Water Filters - Soil Salinity - Hydrosphere
Seawater is water from a sea or ocean has a salinity of about 3.5%, roughly one litre by volume of seawater has approximately 35 grams (1.2 oz) of dissolved salts. Water that has .05% salt is safe to drink. Water with over 3.0% salt will make you sick. PH.
Saline Water or salt water, is water that contains a significant concentration of dissolved salts (mainly NaCl).
Solar Still distills water, using the heat of the Sun to evaporate, cool then collect the water. There are many types of solar still, including large scale concentrated solar stills, and condensation traps (better known as moisture traps amongst survivalists). In a solar still, impure water is contained outside the collector, where it is evaporated by sunlight shining through clear plastic or glass. The pure water vapor condenses on the cool inside surface and drips down, where it is collected and removed. Distillation replicates the way nature makes rain. The sun's energy heats water to the point of evaporation. As the water evaporates, water vapor rises, condensing into water again as it cools and can then be collected. This process leaves behind impurities, such as salts and heavy metals, and eliminates microbiological organisms. The end result is pure distilled water.
Solar Evaporator offers a fresh route to Fresh Water. Self-cleaning device made of wood aims to make small-scale desalination more practical. The design employs a technique known as interfacial evaporation, which are made of thin materials that float on saline water. Absorbing solar heat on top, the evaporators continuously pull up the saline water from below and convert it to steam on their top surface, leaving behind the salt. About a billion people around the world lack access to safe drinking water.
Solar-Powered Water Desalination. A completely passive solar-powered desalination system could provide more than 1.5 gallons of fresh drinking water per hour for every square meter of solar collecting area. Such systems could potentially serve off-grid arid coastal areas to provide an efficient, low-cost water source.
Dewvaporation desalination technology energy efficient tool for freshwater procurement and saline waste stream management.
Desalination Water Treatment Systems
Sea Clearwater Makers
Pure Aqua
Desalination Technologies
Solar Powered Personal Desolenator
Water, water, every where, Nor any drop to drink. Water, Water Everywhere, and Not a Drop to Drink.
Researchers make headway in Desalination Technology. Desalination of brackish waters economically desirable and energy efficient. Sodium-ion batteries theory states that by using electrodes that contain sodium and chloride ions, salt is drawn out and held in a chamber separate from the purified water.
Efficient High-Pressure Desalination. Most desalination plants today use a process called reverse osmosis (RO), which forces water through huge rolls of membranes, leaving the salt behind. One of the most expensive operational challenges for such plants is the fouling of these membranes by microorganisms.
CETO Wave-Energy and Desalinates Water.
SAROS is a wave-powered desalination system which uses the energy in waves to access the nearly limitless supply of water found in our oceans.
Sahara Forest Project aims to provide fresh water, food and renewable energy in hot, arid regions as well as re-vegetating areas of uninhabited desert. This proposal combines saltwater-cooled greenhouses with solar power technologies, either directly using Photovoltaic (PV) or indirectly using concentrated solar power (CSP) and technologies for desert revegetation. It is claimed that these technologies together will create a sustainable and profitable source of energy, food, vegetation and water. Sahara Forest Project.
Aquaporins Desalination Filter
Aquaporin (wiki)
Masdar Institute’s Innovative Wastewater Treatment Technologies to Help Meet Growing Freshwater Demand.
Graphene sieve turns seawater into drinking water. Graphene-oxide membranes developed at the National Graphene Institute have already demonstrated the potential of filtering out small nanoparticles, organic molecules, and even large salts. Until now, however, they couldn’t be used for sieving common salts used in desalination technologies, which require even smaller sieves. Previous research at The University of Manchester found that if immersed in water, graphene-oxide membranes become slightly swollen and smaller salts flow through the membrane along with water, but larger ions or molecules are blocked. The Manchester-based group have now further developed these graphene membranes and found a strategy to avoid the swelling of the membrane when exposed to water. The pore size in the membrane can be precisely controlled which can sieve common salts out of salty water and make it safe to drink.
A New Method for Water Desalination Using Microbial Desalination Cells (PDF)
Ionic 'Solar Cell' could provide On-Demand Water Desalination. Ionic analog to the electronic pn-junction solar cell device that would directly desalinate saltwater upon exposure to sunlight. Bipolar-membrane design for ionic electricity generation. The transport of oppositely charged protons and hydroxides obtained by dissociating water molecules.
Sundrop Farms now produces 15 per cent of the Australian tomato market – all of it grown using seawater.
Seawater Greenhouse - Somaliland Seawater Greenhouse
Filter for an aquarium are critical components of both freshwater and marine aquaria. Aquarium filters remove physical and soluble chemical waste products from aquaria, simplifying maintenance. Furthermore, aquarium filters are necessary to support life as aquaria are relatively small, closed volumes of water compared to the natural environment of most fish.
Illuminating Water Filtration. Researchers using ultrabright X-rays reveal the molecular structure of membranes used in reverse osmosis to purify seawater into drinking water.
Membranes
Membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Biological membranes include cell membranes (outer coverings of cells or organelles that allow passage of certain constituents); nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry (such as chemical plants).
Membrane Technology covers all engineering approaches for the transport of substances between two fractions with the help of permeable membranes. In general, mechanical separation processes for separating gaseous or liquid streams use membrane technology. Metal Ions.
Synthetic Membrane is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. A wide variety of synthetic membranes is known. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. The most of commercially utilized synthetic membranes in separation industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as a choice of driving force define a particular membrane separation process. The most commonly used driving forces of a membrane process in industry are pressure and concentration gradients. The respective membrane process is therefore known as filtration. Synthetic membranes utilized in a separation process can be of different geometry and of respective flow configuration. They can also be categorized based on their application and separation regime. The best known synthetic membrane separation processes include water purification, reverse osmosis, dehydrogenation of natural gas, removal of cell particles by microfiltration and ultrafiltration, removal of microorganisms from dairy products, and Dialysis. Filtering.
Semipermeable Membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by diffusion—or occasionally by more specialized processes of facilitated diffusion, passive transport or active transport. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials thicker than a membrane are also semipermeable. One example of this is the thin film on the inside of the egg. Note that a semipermeable membrane is not the same as a selectively permeable membrane. Semipermeable membrane describes a membrane that allows some particles to pass through (by size), whereas the selectively permeable membrane "chooses" what passes through (size is not a factor).
Nanofiltration is a relatively recent membrane filtration process used most often with low total dissolved solids water such as surface water and fresh groundwater, with the purpose of softening (polyvalent cation removal) and removal of disinfection by-product precursors such as natural organic matter and synthetic organic matter. Nanofiltration is also becoming more widely used in food processing applications such as dairy, for simultaneous concentration and partial (monovalent ion) demineralisation.
Microfiltration is a type of physical filtration process where a contaminated fluid is passed through a special pore-sized membrane to separate microorganisms and suspended particles from process liquid. It is commonly used in conjunction with various other separation processes such as ultrafiltration and reverse osmosis to provide a product stream which is free of undesired contaminants.
Ultrafiltration is a variety of membrane filtration in which forces like pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (103 - 106 Da) solutions, especially protein solutions. Ultrafiltration is not fundamentally different from microfiltration. Both of these separate based on size exclusion or particle capture. It is fundamentally different from membrane gas separation, which separate based on different amounts of absorption and different rates of diffusion. Ultrafiltration membranes are defined by the molecular weight cut-off (MWCO) of the membrane used. Ultrafiltration is applied in cross-flow or dead-end mode. Separation Process.
Electrospray technology is used to create ultra-thin, ultra-smooth polyamide membranes for reverse osmosis. This scalable process allows for better control of a membrane's fundamental properties, avoids the use of chemical baths, and can be applied to a variety of membrane separation processes. Extremely thin polyamide film measuring approximately 1.1 microns thick that has been successfully separated from its underlying substrate, an advantage unique to a new UConn fabrication process and one that makes it easier to characterize the film's properties. Using an additive manufacturing approach employing electrospraying, UConn scientists were able to create ultra-thin, ultra-smooth polyamide membranes that are less prone to fouling and may require less power to move water through them.
Desalination breakthrough could lead to cheaper water filtration. Desalination membranes are inconsistent in density and mass distribution, which can hold back their performance. Uniform density at the nanoscale is the key to increasing how much clean water these membranes can create.
Turning desalination waste into a useful resource. Process developed at MIT could turn concentrated brine into useful chemicals, making desalination more efficient. Sodium hydroxide is not the only product that can be made from the waste brine: Another important chemical used by desalination plants and many other industrial processes is hydrochloric acid, which can also easily be made on site from the waste brine using established chemical processing methods. The chemical can be used for cleaning parts of the desalination plant, but is also widely used in chemical production and as a source of hydrogen. Desalination brine, which can be laden with residual chemicals from the treatment process as well as excess heat, is damaging to the marine environment. Most coastal desalination facilities discharge their waste back into the ocean. ... They are instead concentrated in a hyper-saline brine. The amount of brine generated by the world’s nearly 16,000 desalination plants is 50 percent larger than earlier assumptions. Just four countries on the Arabian Peninsula account for 32 percent of global desalinated water but 55 percent of global brine production. Brine is generally defined as water with a salt concentration higher than 50 parts per thousand, though some brines can be several times saltier. Average salinity of the world’s oceans is roughly 35 parts per thousand. The discharge can also contain precious elements like uranium. This might be enough incentive to turn desal brine from a noxious byproduct into a source of revenue. Or you might use evaporative pools inland to produce commercial road salt for deicing roads.
Water from Trees: Maple Water.
Solvay Process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia-soda process was developed into its modern form by Ernest Solvay during the 1860s. The ingredients for this are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone (from quarries). The worldwide production of soda ash in 2005 has been estimated at 42 million metric tons, which is more than six kilograms (13 lb) per year for each person on Earth. Solvay-based chemical plants now produce roughly three-quarters of this supply, with the remaining being mined from natural deposits. This method superseded the Leblanc process.
Brine is a high-concentration solution of salt in water. In different contexts, brine may refer to salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of solutions used for brining foods) up to about 26% (a typical saturated solution, depending on temperature). Lower levels of concentration are called by different names: fresh water, brackish water, and saline water. Brine naturally occurs on the Earth's surface (salt lakes), crust, and within brine pools on ocean bottom. High-concentration brine lakes typically emerge due to evaporation of ground saline water on high ambient temperatures. Brine is used for food processing and cooking (pickling and brining), for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, and may pose an environmental risk due to its corrosive and toxic effects, so it requires wastewater treatment for proper disposal or further utilization (fresh water recovery).
Salt Water is More Dense than Fresh Water. Density = mass/volume. Increasing the mass by adding salt increases the density. Seawater is a little bit more dense than fresh water so it sinks beneath freshwater. This means that when rivers flow out into the sea the river freshwater floats on top of the sea water. Isohalines are areas in the water that have equal salt concentrations, or salinities. In estuaries, salinity levels are generally highest near the mouth of a river where the ocean water enters, and lowest upstream where fresh water flows in.
Scientists Discover a Vast Reservoir of Semi-Freshwater Hidden Beneath The Ocean. Signals of the water first showed up in the 1970s. Salt water is a more effective conductor of electromagnetic (EM) waves than fresh water, so EM receivers deployed off the coast enabled the researchers to map the extent of the mysterious aquifer. The results, published in a study detailing the first comprehensive attempt to map this giant reservoir, reveal a mostly "continuous submarine aquifer system spans at least 350?km [217 miles] of the US Atlantic coast and contains about 2,800? cubic kilometres of low-salinity groundwater". As for how the aquifer got there, the researchers say it likely happened when vast amounts of fresh meltwater from the last Ice Age got trapped in rocky sediment. Water Research.
Water From Air
Atmospheric Water Generator is a device that extracts water from humid ambient air. Water vapor in the air is condensed by cooling the air below its dew point, exposing the air to desiccants, or pressurizing the air. Unlike a dehumidifier, an AWG is designed to render the water potable. AWGs are useful where pure drinking water is difficult or impossible to obtain, because there is almost always a small amount of water in the air that can be extracted. The two primary techniques in use are cooling and desiccants, which is a hygroscopic substance that is used to induce or sustain a state of dryness or desiccation in its vicinity.
Water Gen Atmospheric Water Generation.
Fresh Water from the Air - Water Generator
WaterSeer extracting Water from the Air
Ecolo Blue
High Volume Water Making Machines
Planets Water 300 Gallons a Day
DIY Water Generator (PDF)
Smart Oasis produces drinking water from the air.
UTEC - Potable Water Generator (youtube)
Skysource is an atmospheric water generator that condenses moisture in the atmosphere and filters it, making fresh drinking water. water xprize.
Zero Mass is a Hydropaneltm that makes drinking water from sunlight and air. Water flows into a 30 liter reservoir where it is mineralized for optimal taste, storing up to 120 standard bottles per 2-panel array. Produces 4 – 10 liters, per 2-panel array of water a day depending on the humidity and sunlight. $2,000.00.
Hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water. In contrast, hydrophobes are not attracted to water and may seem to be repelled by it.
NDB Nano - Edward Linnacre's AirDrop Irrigation (youtube)
Fontus can produce 0.5 quarts (0.5 liters) of water in 1 hour between 86 degrees and 104 degrees Fahrenheit (30 to 40 degrees Celsius) and between 80 percent and 90 percent humidity. A condensator (which functions like a cooler) that is connected to a series of hydrophobic surfaces that repel water. As the bike-mounted gadget takes in air, and these surfaces get cold, you're left with condensation does not include a way to filter out potentially harmful contaminants.
Fog Collection refers to the collection of water from fog using large pieces of vertical canvas to make the fog condense into droplets of water and flow down towards a trough below the canvas, known as a fog fence.
Water from Fog (youtube) - Fog Harvesting
Fog consists of visible cloud water droplets or ice crystals suspended in the air at or near the Earth's surface. Fog can be considered a type of low-lying cloud and is heavily influenced by nearby bodies of water, topography, and wind conditions. In turn, fog has affected many human activities, such as shipping, travel, and warfare.
Warka Water Tower - Ethiopia
Device pulls Water from Dry Air, Powered only by the Sun. Imagine a future in which every home has an appliance that pulls all the water the household needs out of the air, even in dry or desert climates, using only the power of the sun. The prototype, under conditions of 20-30 percent humidity, was able to pull 2.8 liters (3 quarts) of water from the air over a 12-hour period, using one kilogram (2.2 pounds) of MOF. Rooftop tests at MIT confirmed that the device works in real-world conditions.
Device Harvests Water from Desert Air. High-surface-area materials called metal-organic frameworks (MOFs), can extract potable water from even the driest of desert air with relative humidity as low as 10 percent.
Metal-Organic Framework are compounds consisting of metal ions or clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. They are a subclass of coordination polymers, with the special feature that they are often porous. The organic ligands included are sometimes referred to as "struts", one example being 1,4-benzenedicarboxylic acid (BDC). More formally, a metal–organic framework is a coordination network with organic ligands containing potential voids. A coordination network is a coordination compound extending, through repeating coordination entities, in one dimension, but with cross-links between two or more individual chains, loops, or spiro-links, or a coordination compound extending through repeating coordination entities in two or three dimensions; and finally a coordination polymer is a coordination compound with repeating coordination entities extending in one, two, or three dimensions. In some cases, the pores are stable during elimination of the guest molecules (often solvents) and could be used for the storage of gases such as hydrogen and carbon dioxide. Other possible applications of MOFs are in gas purification, in gas separation, in catalysis, as sensors and as supercapacitors. Reticular Chemistry is concerned largely (but in principle, not exclusively) with the synthesis and properties of metal-organic frameworks (MOFs), particularly those in which the components are linked by strong bonds such as occur in metal carboxylates.
Kilogram of MOF produces 200 ml of water per day/night cycle from dry air, using only solar energy.
Water Harvesting Inc. MOF-303 is based on aluminum cations linked by 3,5-pyrazoledicarboxylic acid units.
Covalent Organic Framework are two-dimensional and three-dimensional organic solids with extended structures in which building blocks are linked by strong covalent bonds. COFs are porous and crystalline and are made entirely from light elements (H, B, C, N, and O) that are known to form strong covalent bonds in well-established and useful materials such as diamond, graphite, and boron nitride. Preparation of COF materials from molecular building blocks would provide covalent frameworks that could be functionalized into lightweight materials for diverse applications.
Evaporation - Dew Point - Rain - Snow
Condensation is the change of the physical state of matter from gas phase into liquid phase, and is the reverse of evaporation. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapour to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition, or desublimation, Sublimation (phase transition), which is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase.
Pesticides - Insecticides - Herbicides
 Pesticide are chemicals that are meant to kill
Animals.
Pesticide are chemicals that are meant to kill
Animals.Systemic Pesticides are chemicals that are actually absorbed by a plant when applied to seeds, soil, or leaves. The chemicals then circulate through the plant's tissues, killing the insects that feed on them, as well as the humans who eat the plant that's infused with the poison pesticide. Organic Pesticides.
Insecticide is a substance used to kill insects.
Herbicide or weed killers, are chemical substances used to control unwanted plants.
Pesticide Action Network (panna)
Pesticide Database - Toxins - Sressors
Beyond Pesticides - Pollution - GMO
Integrated Pest Management is a broad-based approach that integrates practices for economic control of pests.
Soil Testing - Fertilizers
Neonicotinoid are a class of neuro-active insecticides chemically similar to nicotine.
Bees - Spray Safe - Organic Pesticides
Pesticide Watch
Pheromone Trap is a type of insect trap that uses pheromones to lure insects.
Rootworm larvae can destroy significant percentages of corn. Lawns - Yards.
Food Pesticide List - Foods that Have Pesticides (Info-Graph Image)
Eco Smart Safe Pesticides - Shoppers Guide for Pesticides (pdf)
Persistent Organic Pollutant are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes. Because of their persistence, POPs bioaccumulate with potential adverse impacts on human health and the environment. The effect of POPs on human and environmental health was discussed, with intention to eliminate or severely restrict their production, by the international community at the Stockholm Convention on Persistent Organic Pollutants in 2001. Many POPs are currently or were in the past used as pesticides, solvents, pharmaceuticals, and industrial chemicals. Although some POPs arise naturally, for example volcanoes and various biosynthetic pathways, most are man-made via total synthesis.
Sugarcane - Sugar
Atrazine is an herbicide of the triazine class. Atrazine is used to prevent pre- and postemergence broadleaf weeds in crops such as maize (corn) and sugarcane and on turf, such as golf courses and residential lawns. An effective way to eliminate atrazine and its by-products in surface water.
Bromomethane is a pesticide being phased out by most countries in the early 2000.
Glyphosate is a broad-spectrum systemic herbicide and crop desiccant. Monsanto's Roundup - False Advertising.
Heptachlor is Linked To Parkinson's Disease.
Methyl Isocyanate is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane, methyl carbylamine, and MIC. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives. As a highly toxic and irritating material, it is extremely hazardous to human health. It was the principal toxicant involved in the Bhopal disaster, which killed nearly 2,259 people initially and officially 3,787 people in total. This family of organic molecules is involved in the synthesis of peptides and amino acids, which, in the form of proteins, are the biological basis for life as we know it."
Chlorpyrifos is a crystalline organophosphate insecticide, acaracide and miticide. Chlorpyrifos exposure has been linked to neurological effects, persistent developmental disorders and autoimmune disorders. Exposure during pregnancy retards the mental development of children, and most home use was banned in 2001 in the U.S. In agriculture, it is "one of the most widely used organophosphate insecticides" in the United States, according to the United States Environmental Protection Agency (EPA), and before being phased out for residential use was one of the most used residential insecticides. On March 29, 2017, EPA Administrator Scott Pruitt denied a petition to ban chlorpyrifos.
Seed Treatment or seed dressing is when seeds are treated with a chemical, like an antimicrobial, fungicidal or insecticide prior to planting. It is usual to add colour to make treated seed less attractive to birds, and easier to see and clean up in the case of an accidental spillage. Specialist machinery is required to safely and efficiently apply the chemical to the seed. A seed coating is a thicker form of covering of seed and may contain fertiliser, growth promoters and or seed treatment as well as an inert carrier and a polymer outer shell. The term "seed dressing" is also used to refer to the process of removing chaff, weed seeds and straw from a seed stock.
Overuse of herbicides costing UK economy £400 million per year. Widespread use of herbicides leading to resistant black-grass is costing UK millions in profit.
Chemical Warfare - Poisoning People for Profit
Chemical Warfare involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from nuclear warfare and biological warfare, which together make up NBC, the military acronym for nuclear, biological, and chemical (warfare or weapons), all of which are considered "weapons of mass destruction" (WMDs). None of these fall under the term conventional weapons which are primarily effective due to their destructive potential.
Toxins in Products - Pharmaceutical Drugs in City Water - Food Additives - Pollution - Treason - War Criminals
Humans and the environment are just collateral damage and an externality of doing business in the name of greed. Some manufacturers who sell pesticides and insecticides have no third party testing to confirm how safe a product is. They do their own testing so they can cherry pick data and then manipulate weak rules and regulations to sell their poisonous product on the market. There is no transparency or accountability, just poisoned water, plants, soil, food, air and poisoned minds.
Biological Warfare is the use of biological toxins or infectious agents such as bacteria, viruses, and fungi with the intent to kill or incapacitate humans, animals or plants as an act of war. Biological weapons (often termed "bio-weapons", "biological threat agents", or "bio-agents") are living organisms or replicating entities (viruses, which are not universally considered "alive") that reproduce or replicate within their host victims. Entomological (insect) warfare is also considered a type of biological weapon. This type of warfare is distinct from nuclear warfare and chemical warfare, which together with biological warfare make up NBC, the military initialism for nuclear, biological, and chemical warfare using weapons of mass destruction (WMDs). None of these is a conventional weapon, which are deployed primarily for their explosive, kinetic, or incendiary potential.
Bioterrorism is terrorism involving the intentional release or dissemination of biological agents. These agents are bacteria, viruses, fungi, or toxins, and may be in a naturally occurring or a human-modified form, in much the same way in biological warfare. Pesticides.
Weapon of Mass Destruction is a nuclear, radiological, chemical, biological, or any other weapon that can kill and bring significant harm to numerous humans or cause great damage to human-made structures (e.g., buildings), natural structures (e.g., mountains), or the biosphere.
Biological Agent is a bacterium, virus, protozoan, parasite, or fungus that can be used purposefully as a weapon in bioterrorism or biological warfare (BW). In addition to these living and/or replicating pathogens, Toxins and biotoxins are also included among the bio-agents. More than 1,200 different kinds of potentially weaponizable bio-agents have been described and studied to date. Biological agents have the ability to adversely affect human health in a variety of ways, ranging from relatively mild allergic reactions to serious medical conditions, including serious injury, as well as serious or permanent disability or even death. Many of these organisms are ubiquitous in the natural environment where they are found in water, soil, plants, or animals. Bio-agents may be amenable to "weaponization" to render them easier to deploy or disseminate. Genetic modification may enhance their incapacitating or lethal properties, or render them impervious to conventional treatments or preventives. Since many bio-agents reproduce rapidly and require minimal resources for propagation, they are also a potential danger in a wide variety of occupational settings. (A biological agent—also called bio-agent, biological threat agent, biological warfare agent, biological weapon, or bioweapon).
Food Denial by Poisoning the Land, Air, Food and Water. A systematic mass murder. Your toxic cocktail is ready, time to Drink the Kool-Aid, without your consent or knowledge.
Silent Spring is an environmental science book by Rachel Carson that was published on September 27, 1962, documenting the adverse environmental effects caused by the indiscriminate use of pesticides. Carson accused the chemical industry of spreading disinformation, and public officials of accepting the industry's marketing claims unquestioningly. The book led to a nationwide ban on DDT for agricultural uses, and helped to inspire an environmental movement that led to the creation of the U.S. Environmental Protection Agency.
Food Tampering is the intentional contamination of a food product, with the intent to cause harm to the consumer or to a private company. Food tampering may affect any part of the food product, such as the product itself, or it can affect the packaging and the label. Tampering can refer to many forms of sabotage but the term is often used to mean intentional modification of products in a way that would make them harmful to the consumer. This threat has prompted manufacturers to make products that are either difficult to modify or at least difficult to modify without warning the consumer that the product has been tampered with. Since the person making the modification is typically long gone by the time the crime is discovered, many of these cases are never solved. The crime is often linked with attempts to extort money from the manufacturer, and in many cases no contamination to a product ever takes place. Fraud is sometimes handled as a matter of civil law, but actual modification of products is almost always a matter of criminal law. Food Safety.
Infrastructure Security is the security provided to protect infrastructure, especially critical infrastructure, such as airports, highways rail transport, hospitals, bridges, transport hubs, network communications, media, the electricity grid, dams, power plants, seaports, oil refineries, and water systems. Infrastructure security seeks to limit vulnerability of these structures and systems to sabotage, terrorism, and contamination. Big 5 Needs.
National Security is protection against military attack, and national security is now widely understood to include non-military dimensions, including the security from terrorism, crime, economic security, energy security, environmental security, food security, cyber security etc. Similarly, national security risks include, in addition to the actions of other nation states, action by violent non-state actors, narcotic cartels, and multinational corporations, and also the effects of natural disasters.
Operation Ranch Hand herbicidal warfare program during the war called "Operation Trail Dust". Ranch Hand involved spraying an estimated 20 million U.S. gallons (76,000 m3) of defoliants and herbicides over rural areas of South Vietnam in an attempt to deprive the Viet Cong of food and vegetation cover. Areas of Laos and Cambodia were also sprayed to a lesser extent. Nearly 20,000 sorties were flown between 1961 and 1971.
Herbicidal Warfare is the use of substances primarily designed to destroy the plant-based ecosystem of an area.
Organization for the Prohibition of Chemical Weapons
Scorched Earth is a government or corporate policy used as a military strategy that targets anything that might be useful to people while advancing through or withdrawing from an area. Any assets that could be used by people may be targeted, for example food sources, transportation, communications, industrial resources, and even the people in the area.
Food Whistleblower Protecting Food. Empowering Whistleblowers.
World Governments signed agreements to end Crimes against Humanity.
Association between two commonly used agrochemicals (paraquat and maneb) and Parkinson's disease.
Monsanto is finally found Guilty, again. Thank you to the 12 jurors who listened attentively and critically, during long days of testimony, then deliberated with care, and ultimately did the right thing. Thank you to the lawyers who invested countless hours in investigative work and trial preparation, and who argued rationally and intelligently on behalf of the plaintiff, science and ethics. Thank you to those media outlets and advocacy organizations who covered the case, pored over the “Monsanto Papers” and took seriously their obligation to inform the public. But most of all, we owe a huge debt of gratitude to Dewayne “Lee” Johnson, the plaintiff in the Dewayne Johnson v. Monsanto case. For his persistence in getting to the bottom of what caused his non-Hodgkin lymphoma. For is bravery in going up against one of the most powerful corporations in the world. And for his refusal to give up, no matter the toll on his family, and on his failing health. The jury’s decision was unanimous: Monsanto was guilty of manufacturing and selling a product that caused Johnson’s cancer. What’s more, the company knew its product could cause cancer—and yet it intentionally hid that fact from Johnson and the public. The answer to question after question, about whether Roundup caused Johnson’s cancer, whether the product poses a danger to consumers, whether the product’s risk was known or knowable? Yes. Yes. Yes.
The Monsanto Papers (youtube)
Time to Face the Music. You can run, but you can't hide. You can lie, but you can't change the truth.
Farmed Norwegian Salmon World’s Most Toxic Food (youtube) - UBM Media - Baltic Sea is one of the most polluted seas in the world. Ethoxyquin is used as a preservative in some pet foods to slow the development of rancidity of fats. Health consequences from these effects are not known.
Food Fraud can include deceptive labeling, the lack of food purity, and more. According to the U.S. Pharmacopeial Convention, a critically important entity protecting food and drug purity, the amount of fake ingredients has increased by 60 percent in the last year. Food Fraud Initiative - European Food Safety Authority - False Advertising.
Counterfeit Consumer Goods is the intentional adulteration of food with cheaper ingredients for economic gain.
Body Burden - Neurotoxins
Adverse Health Effect is defined as the causation, promotion, facilitation and/or exacerbation of a structural and/or functional abnormality, with the implication that the abnormality produced has the potential of lowering the quality of life, contributing to a disabling illness, or leading to a premature death.
Body affects the Mind and vise versa. Disease Burden.
Endocrine Disruptor are chemicals that, at certain doses, can interfere with endocrine or hormone systems. These disruptions can cause cancerous tumors, birth defects, and other developmental disorders. Any system in the body controlled by hormones can be derailed by hormone disruptors.
Neurotoxin are toxins that are poisonous or destructive to nerve tissue (causing neurotoxicity). Brain Damage.
Neurotoxicity is when exposure to natural or artificial toxic substances, which are called neurotoxins, alters the normal activity of the nervous system in such a way as to cause damage to nervous tissue. This can eventually disrupt or even kill neurons, key cells that transmit and process signals in the brain and other parts of the nervous system.
Neurological Disorder are diseases of the brain, spine and the nerves that connect them. There are more than 600 diseases of the nervous system, such as brain tumors, epilepsy, Parkinson's disease and stroke as well as less familiar ones such as frontotemporal dementia.
Genotoxicity describes the property of chemical agents that damages the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, whereas not all genotoxic substances are mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.
1.1 Billion Pounds of Dangerous Pesticides are used in the U.S. Annually. Apoptosis - Cell Death.
Biocide is a diverse group of poisonous substances including preservatives, insecticides, disinfectants, and pesticides used for the control of organisms that are harmful to human or animal health or that cause damage to natural or manufactured products. A chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism by chemical or biological means.
Hormesis is any process in a cell or organism that exhibits a biphasic response to exposure to increasing amounts of a substance or condition. Within the hormetic zone, there is generally a favorable biological response to low exposures to toxins and other stressors.
Stressor is a chemical or biological agent, environmental condition, external stimulus or an event that causes stress to an organism. Psychologically speaking, a stressor can be events or environments that an individual would consider demanding, challenging, and or threaten the individual's safety. Stressors have physical, chemical and mental responses inside of the body. Physical stressors produce mechanical stresses on skin, bones, ligaments, tendons, muscles and nerves that cause tissue deformation and in extreme cases tissue failure. Chemical stresses also produce biomechanical responses associated with metabolism and tissue repair. Physical stressors may produce pain and impair work performance. Chronic pain and impairment requiring medical attention may result from extreme physical stressors or if there is not sufficient recovery time between successive exposures. Stressors may also affect mental function and performance. One possible mechanism involves stimulation of the hypothalamus, CRF (corticotropin release factor) -> pituitary gland releases ACTH (adrenocorticotropic hormone) -> adrenal cortex secretes various stress hormones (e.g., cortisol) -> stress hormones (30 varieties) travel in the blood stream to relevant organs, e.g., glands, heart, intestines -> flight-or-fight response. Between this flow there is an alternate path that can be taken after the stressor is transferred to the hypothalamus, which leads to the sympathetic nervous system. After which, the adrenal medulla secretes epinephrine. Mental and social stressors may affect behavior and how individuals respond to physical and chemical stressor.
Horizontal Gene Transfer is the movement of genetic material between unicellular and/or multicellular organisms other than by the ("vertical") transmission of DNA from parent to offspring. HGT is an important factor in the evolution of many organisms.
Chronic Kidney Disease is progressive loss in kidney function over a period of months or years.
Coeliac Disease is a long term autoimmune disorder primarily affecting the small intestine that occurs in people who are genetically predisposed.
Non-Hodgkin Lymphoma is a group of blood cancers that includes all types of lymphoma except Hodgkin's lymphomas. Symptoms include enlarged lymph nodes, fever, night sweats, weight loss, and tiredness. Other symptoms may include bone pain, chest pain, or itchiness. Some forms are slow growing while others are fast growing.
Congenital Disorder is a condition existing at or before birth regardless of cause.
Insecticide Runoff Maps and Charts - Erosion
Slow Poisoning of India (youtube)
Our Daily Poison (2011) (1:52) - An in-depth investigation into everyday products and the system charged with regulating them. Robin digs through the United States Food and Drug Administration (FDA) and the European Food.
Pesticides, additives, food coloring, packaging. Scientific studies that have been ignored, and some studies they use are flawed, no reasoning.
What is Natural? - Healthy?
Chemical Exposure Linked to Health Care Costs
Cancer took more than 8.7 million years of life and $94.4 billion in lost earnings among people ages 16 to 84 in the United States in 2015. Population of US Cancer Survivors Grows to Nearly 17 Million. Many Cancer Survivors Struggle to Pay For Care.
Plastics pose threat to human health, report shows. Plastics contain and leach hazardous chemicals, including endocrine-disrupting chemicals (EDCs) that threaten human health. EDCs are chemicals that disturb the body's hormone systems and can cause cancer, diabetes, reproductive disorders, and neurological impairments of developing fetuses and children. The report describes a wealth of evidence supporting direct cause-and-effect links between the toxic chemical additives in plastics and specific health impacts to the endocrine system. Conservative estimates point to more than a thousand manufactured chemicals in use today that are EDCs. Known EDCs that leach from plastics and threaten health include bisphenol A and related chemicals, flame retardants, phthalates, per- and polyfluoroalkyl substances (PFAS), dioxins, UV-stabilizers, and toxic metals such as lead and cadmium. Plastic containing EDCs is used extensively in packaging, construction, flooring, food production and packaging, cookware, health care, children's toys, leisure goods, furniture, home electronics, textiles, automobiles and cosmetics. Key findings in the report include: One hundred and forty four chemicals or chemical groups known to be hazardous to human health are actively used in plastics for functions varying from antimicrobial activity to colorants, flame retardants, solvents, UV-stabilizers, and plasticizers. Exposure can occur during the entire life span of plastic products, from the manufacturing process to consumer contact, recycling, to waste management and disposal. EDC exposure is a universal problem. Testing of human samples consistently shows nearly all people have EDCs in their bodies. Microplastics contain chemical additives, which can leach out of the microplastic and expose the population. They can also bind and accumulate toxic chemicals from the surrounding environment, such as seawater and sediment, functioning as carriers for toxic compounds. Bioplastics/biodegradable plastics, promoted as more ecological than plastics, contain similar chemical additives as conventional plastics and also have endocrine-disrupting effects.
COVID-19 spike proteins may cause neurological issues. COVID-19 virus enters the brain. The spike proteins S1 protein alone can cause brain fog. Since the spike protein enters the brain, the virus also is likely to cross into the brain. The S1 protein likely causes the brain to release cytokines and inflammatory products. The intense inflammation caused by the COVID-19 infection is called a cytokine storm. The immune system, upon seeing the virus and its proteins, overreacts in its attempt to kill the invading virus. The infected person is left with brain fog, fatigue and other cognitive issues. S1 protein in SARS-CoV2 and the gp 120 protein in HIV-1 function similarly. glycoproteins -- proteins that have a lot of sugars on them, hallmarks of proteins that bind to other receptors. Both these proteins function as the arms and hand for their viruses by grabbing onto other receptors. Both cross the blood-brain barrier and S1, like gp120, is likely toxic to brain tissues.
Body Burden Testing
Bio-Monitoring is the measurement of the body burden of toxic chemical compounds, elements, or their metabolites, in biological substances.
Years of Potential Life Lost - Disease Burden
What is a Body Burden? - Cocktail Effect - Damage to the Brain and Body
Acceptable Daily Intake is a measure of the amount of a specific substance (originally applied for a food additive, later also for a residue of a veterinary drug or pesticide) in food or drinking water that can be ingested (orally) on a daily basis over a lifetime without an appreciable health risk. ADIs are expressed usually in milligrams (of the substance) per kilograms of body weight per day.
Heavy Metal Test Analysis (PDF)
Infectious Diseases Emergency Preparedness Plan
Household Chemicals Cancer Concern
National Industrial Chemicals Notification and Assessment Scheme is designed to help protect workers, the public and the environment from the harmful effects of industrial chemicals. It does so by making risk assessment and safety information on chemicals widely available and providing recommendations for their safe use. NICNAS also informs importers and manufacturers of their legal responsibilities.
Toxins and Child Birth - Vitals
Processed Food Warnings - Food Safety - Natural
Economic Injury Level (EIL) The level of pest infestation below which the cost of further reducing the pest population exceeds the additional revenue or value of other benefits such reduction would achieve.
Pollution - Sanitation
Consumer Confidence Report - Pacific Institute
97% of water on earth is undrinkable. (Desalination)
Why are the Feds allowing industries to Pollute the Nations Underground Water Supply?
Environmental Protection Agency. Since 2004, the water provided to more than 49 million Americans has contained illegal concentrations of chemicals like arsenic or radioactive substances like uranium, as well as dangerous bacteria often found in sewage. But I still don't buy bottled water because that just adds to the problem. I use a water filter and a reusable water bottle. We definitely have to make the EPA more responsible for their failures if we want things to improve.
Organic Pesticides - Safe and Healthy
Green Pesticides is a broad-based approach that integrates practices for economic control of pests. IPM aims to suppress pest populations below the economic injury level, which is the smallest number of insects or amount of injury that will cause yield losses equal to the insect management costs. Economic threshold is the density of a pest at which a control treatment will provide an economic return. The pest density at which management action should be taken to prevent an increasing pest population from reaching the economic injury level, meaning not enough profit for greedy corporations who are already getting subsidized. If society is going to subsidize a farmer, it should be a farmer who uses safe pesticides and safe herbicides.
Integrated Pest Management (wiki)
Bio-Pesticide is a contraction of biological pesticides, which include several types of pest management intervention through predatory, parasitic, or chemical relationships. The term has been associated historically with biological control - and by implication - the manipulation of living organisms.
Organic Pest Control
Biological Pest Control is a method of controlling pests such as insects, mites, weeds and plant diseases using other organisms. It relies on predation, parasitism, herbivory, or other natural mechanisms, but typically also involves an active human management role. It can be an important component of integrated pest management (IPM) programs.
A Guide to Organic Pest-Free Gardening - Info-Graph (image)
Organically Grown Food Pesticides
Learn to Grow Organic Food
Non-Pesticide Management describes various pest-control techniques which do not rely on pesticides. It is used in organic production of foodstuff, as well as in other situations in which the introduction of toxins is undesirable. Instead of the use of synthetic toxins, pest control is achieved by biological means.
Plant Diseases - Fertilizers
Organic Weed Spray: 1 gallon of water, 2 cups of Epson salt, 1/4 cup of dish soap (original blue dawn)
Weed killer: This is as easy as filling a spray bottle with white vinegar and adding a teaspoon of dish soap. Be careful when spraying this solution because it can kill your plants too.
Insect repellent: In a large pot, mix two heads of crushed garlic, 3 cups of crushed mint leaves, 2 teaspoons of cayenne pepper and 12 cups of water, and bring to a boil. Let the mixture sit overnight and then strain it into a couple of spray bottles, adding a few squirts of dish soap to each. This should yield 12 cups of liquid..
Biodynamics Non-Chemical Agricultural
Biodynamic Agriculture is a form of alternative agriculture very similar to organic farming, but it includes various esoteric concepts.
Electronic Pest Control is the use of any of the several types of electrically powered devices designed to repel or eliminate pests, usually rodents or insects.
Sound waves as a Pesticide
Flowers Instead of Pesticides (PDF)
Hedge is a line of closely spaced shrubs and tree species, planted and trained to form a barrier or to mark the boundary of an area, such as between neighboring properties.
Crops that kill pests by shutting off their genes. Plants are among many eukaryotes that can 'turn off' one or more of their genes by using a process called RNA interference to block protein translation. Researchers are now weaponizing this by engineering crops to produce specific RNA fragments that, upon ingestion by insects, initiate RNA interference to shut down a target gene essential for life or reproduction, killing or sterilizing the insects.
Impact of weather and well-timed cultural management techniques on organic weed control. Careful rotary hoeing can improve crop competitiveness by reducing and delaying weed emergence relative to the crop. Delayed planting allows time for destruction of early emerging weeds and reduces emerged weed populations. Diverse crop rotations can dampen and diversify weed populations and improve soil fertility.
Farm Cat is a domestic cat, usually of mixed breed, that lives primarily out-of-doors, in a feral or semi-feral condition on agricultural properties, usually sheltering in outbuildings. They eat assorted vermin such as rodents and other small animals that
live in or around outbuildings and farm fields. The need for the farm cat may have been the original reason cats were domesticated, to keep rodents from consuming or contaminating grain crops stored for later human consumption. They are still
commonly kept for their effectiveness at controlling undesired vermin found on farms and ranches, which would otherwise eat or contaminate crops, especially grain or feed stocks.
Cat Predation on Wildlife. Cats hunt small prey, and both feral and domesticated cats prey on wildlife. This is sometimes seen as a desirable phenomenon, such as in the case of barn cats and other cats kept for the purposes of pest control. In other cases, the effect of cats on wildlife is met with concern. Cats that live in the wild or Domestic indoor pets allowed to roam outdoors kill from 1.4 billion to as many as 3.7 billion birds in the continental U.S. each year, says a new study that escalates a decades-old debate over the feline threat to native animals.
Sustainability
Worm pheromones protect major crops. Protecting crops from pests and pathogens without using toxic pesticides has been a longtime goal of farmers. Researchers have found that compounds from an unlikely source - microscopic soil roundworms - could achieve this aim. These compounds helped protect major crops from various pathogens, and thus have potential to save billions of dollars and increase agricultural sustainability around the world.
Films about Water
Blue Gold: World Water Wars (wiki) - Website
Poisoned Water - Frontline (2009 PBS)
Flow: For Love of Water (youtube)
TROM - 2.11 Food and Water (youtube)
Tapped the Film - Website - Tap water costs 10,000 times less then bottled water.
Bolivia Water Wars (youtube)
Cochabamba Protests (wiki)
Water Privatization is the private ownership of water distribution and sanitation.
The Great Culling: Our Water (youtube)
Fluoride Alert
FIRE WATER: Australia's Industrial Fluoridation Disgrace (youtube)
Water Fluoridation Videos
Fluoride Facts - Fluoride in Water - Fluoride Poisoning
TEDxPotomac - Alexandra Cousteau - Connected by Water (youtube)
More Documentaries - Dams
Water Wars 12/01/2014 | 55 min. - The nation of Bangladesh is prey to every threat from water known to man.
Position Based Fluids Demonstration (youtube)
City Water - Public Water Supply
 Public Water System
refers to certain utilities and organizations providing drinking water.
Public Water System
refers to certain utilities and organizations providing drinking water.
Water Supply is the provision of water by public utilities commercial organizations, community endeavors or by individuals, usually via a system of pumps and pipes. Irrigation is covered separately.
History of Municipal Drinking Water (wiki) - Filtration - Wells
Tap Water Executive Summary (EWG)
Pipeline Transport is the transportation of goods or material through a pipe. The latest data from 2014 gives a total of slightly less than 2,175,000 miles (3,500,000 km) of pipeline in 120 countries of the world. The United States had 65%, Russia had 8%, and Canada had 3%, thus 75% of all pipeline were in these three countries.
Infrastructure - Waste Management - Fluid Mechanics
A better way to detect underground water leaks. Researchers propose a new way to locate water leaks within the tangle of aging pipes found beneath many cities. The improvement could save time, money and billions of gallons of water. The researchers built upon a technique known as the water hammer test -- the industry standard for predicting the location of leaks. The test involves suddenly shutting off flow through a pipe and using sensors to gather data about how the resulting shock wave, or "water hammer," propagates. Tartakovsky and Alawadhi propose a new way to assimilate this data into a mathematical model to narrow down the location of a leak.
Aqueduct is a watercourse constructed to convey water. In modern engineering, the term aqueduct is used for any system of pipes, ditches, canals, tunnels, and other structures used for this purpose. The term aqueduct also often refers specifically to a bridge on an artificial watercourse. Irrigation - Dry Land Farming.
Aqueduct bridge is for conveying water, called aqueducts or water bridges are constructed to convey watercourses across gaps such as valleys or ravines.
Viaduct is a bridge composed of several small spans for crossing a valley, gorge, marshland or forming a flyover.
Aquifer is an underground layer of water-bearing permeable rock, rock fractures or unconsolidated materials (gravel, sand, or silt) from which groundwater can be extracted using a water well. Fracking.
Ogallala Aquifer is a shallow water table aquifer surrounded by sand, silt, clay and gravel located beneath the Great Plains in the United States. One of the world's largest aquifers, it underlies an area of approximately 174,000 sq mi (450,000 km2) in portions of eight states (South Dakota, Nebraska, Wyoming, Colorado, Kansas, Oklahoma, New Mexico, and Texas).
Groundwater Recharge is a hydrologic process where water moves downward from surface water to groundwater. Recharge is the primary method through which water enters an aquifer. This process usually occurs in the vadose zone below plant roots and is often expressed as a flux to the water table surface. Recharge occurs both naturally (through the water cycle) and through anthropogenic processes (i.e., "artificial groundwater recharge"), where rainwater and or reclaimed water is routed to the subsurface.
Scientists map huge undersea fresh-water aquifer trapped in porous sediments lying below the salty ocean off U.S.
Northeast. The study suggests that such aquifers probably lie off many other coasts worldwide, and could provide desperately needed water for arid areas that are now in danger of running out.
Reservoir is an enlarged natural or artificial lake, storage pond or impoundment created using a dam or lock to store water. Reservoirs can be created by controlling a stream that drains an existing body of water. They can also be constructed in river valleys using a dam. Alternately, a reservoir can be built by excavating flat ground and/or constructing retaining walls and levees.
Lakes - Water EPA
Safety Concerns - Drinking Water Quality Alerts (EPA)
Water Treatment is any process that makes water more acceptable for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use.
City Water Treatment Process - Drinking Water Treatment Plant (youtube)
Water Purification is the process of removing undesirable chemicals, biological contaminants, suspended solids and gases from contaminated water. The goal is to produce water fit for a specific purpose. Most water is disinfected for human consumption (drinking water), but water purification may also be designed for a variety of other purposes, including fulfilling the requirements of medical, pharmacological, chemical and industrial applications. The methods used include physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination and the use of electromagnetic radiation such as ultraviolet light. Purifying water may reduce the concentration of particulate matter including suspended particles, parasites, bacteria, algae, viruses, fungi, as well as reducing the amount of a range of dissolved and particulate material derived from the surfaces that come from runoff due to rain.
Water Treatment methods may unintentionally generate Harmful Chemicals. Water treatment process converts commonly used chemicals into harmful compounds that affect cellular function and metabolism, study finds.
Sanitation refers to public health conditions related to clean drinking water and adequate treatment and disposal of human excreta and sewage. Preventing human contact with feces is part of sanitation, as is hand washing with soap. Sanitation system aim to protect human health by providing a clean environment that will stop the transmission of disease, especially through the fecal-oral route. For example, diarrhea, a main cause of malnutrition and stunted growth in children, can be reduced through sanitation. There are many other diseases which are easily transmitted in communities that have low levels of sanitation, such as ascariasis (a type of intestinal worm infection or helminthiasis), cholera, hepatitis, polio, schistosomiasis, trachoma, to name just a few.
American Water Works Association
Nebraska Water Center | University of Nebraska, Lincoln
Ebb and Flow are two phases of the tide or any similar movement of water. The ebb is the outgoing phase, when the tide drains away from the shore; and the flow is the incoming phase when water rises again. The terms are also common in figurative use.
Water Purification Process (City)
The first step of water purification is called Coagulation. Like blood forming a scab, the alum helps to chunk up the organic material in the water, so it can fall to the bottom of the tank. It works because alum—also known as Aluminum Sulfate—has a positive charge, whereas the organic gunk floating in the water tends to be negatively charged. They stick together and form a solid, which falls out of the water in a process called Sedimentation. Then the now clear water goes through the filtration step, where it wends its way through several layers of sand, gravel, and charcoal. This removes much of the smaller particles. In the last step, it’s treated with Chloramines to kill bacteria and other microorganisms, giving the water its faintly stinky swimming pool smell. The techniques to treat its drinking water are used around the world, and those methods have proven successful so far.
EPA Water Treatment Guidelines - Waste Water Treatment
Pharmaceutical Drugs in Public Drinking Water
Sanitary Sewer Overflow is a condition in which untreated sewage is discharged from a sanitary sewer into the environment prior to reaching sewage treatment facilities. When caused by rainfall it is also known as wet weather overflow. It is primarily meaningful in developed countries, which have extensive treatment facilities. Frequent causes of SSO spills include: Blockage of sewer lines. Infiltration/Inflow of excessive stormwater into sewer lines during heavy rainfall. Malfunction of pumping station lifts or electrical power failure. Broken sewer lines. EPA.
Storm Water Management - Landscaping - Erosion
Combined Sewer is a sewage collection system of pipes and tunnels designed to also collect surface runoff. Combined sewers can cause serious water pollution problems during combined sewer overflow (CSO) events when wet weather flows exceed the sewage treatment plant capacity. This type of sewer design is no longer used in building new communities (because current design separates sanitary sewers from runoff), but many older cities continue to operate combined sewers.
Toilets (types)
Combined Sewage Overflows (CSO's)
City Water Infrastructure
They are supposed to test for a wide gamut of potentially harmful contaminants. Some of these include naturally occurring microorganisms, such as Cryptosporidium and Coliform Bacteria; metals such as Barium, Copper, Lead; and Herbicides such as atrazine. Drinking water treatment plants in the U.S. are supposed to publish a water quality report every year, noting contaminates that were present above the detection level. In total, the EPA requires drinking water treatment plants to test for almost 90 different contaminants. But noticeably absent from this list are any type of drug or pharmaceutical.
Drinking Water Treatment Plant (youtube)
3D data visualization and mapping of the water infrastructure of San Francisco
2.5 billion people around the world currently lack access to improved sanitation, and 27 percent of urban dwellers in developing nations do not have access to piped water in their homes. Every day, around 2 million tons of human waste are disposed of in water channels.
The World Health Organization reports that 3.4 million people—mainly children—die each year from water-related diseases like cholera, dysentery, or typhoid.
The EPA estimates anywhere from 23,000 to 75,000 overflows of sanitary-sewer systems each year in the U.S.
The right infrastructure becomes critical in preserving water quality and preventing a shortage of clean drinking water. Unfortunately, most of the technology employed by cities today lags behind the latest innovations.
Toilets - Water Filters
Microwave treatment is an inexpensive way to clean heavy metals from treated sewage. A team of researchers studying new methods to remove toxic heavy metals from biosolids -- the solid waste left over after sewage treatment -- found the key is a brief spin through a microwave. The method removed three times the amount of lead from biosolids compared to conventional means and could reduce the total cost of processing by more than 60 percent, making it a possible engineering solution to help produce fertilizer and allow more people to live with clean soil and water.
Wells
Water Well is an excavation or structure created in the ground by digging, driving, boring, or drilling to access groundwater in underground aquifers. The well water is drawn by a pump, or using containers, such as buckets, that are raised mechanically or by hand. Wells were first constructed at least eight thousand years ago and historically vary in construction from a simple scoop in the sediment of a dry watercourse to the stepwells of India, the qanats of Iran, and the shadoofs and sakiehs of India. Placing a lining in the well shaft helps create stability and linings of wood or wickerwork date back at least as far as the Iron Age.
Well is a hole that is dug into the Earth to get water. A qanat is an ancient complex water well system used in the Middle East. Wells can be as simple as a hole that a bucket on a rope can be lowered into, or very complex with pipes and high-powered pumps to get the water out. Most cities that are not close to fresh water lakes or rivers get their water from wells. It is important to be careful what rubbish is put into the ground near a well. If something toxic is put in the ground, it could end up in the water from the well and make people sick. Water is a problem for many African countries. Many charities are helping to build wells in local villages to help stop lengthy travel to distant water supply. A well only works if underneath there is an aquifer which feeds it.
Fresh Water Well
Thirst Project
Private Water Wells
Human Powered Well Pumps
Well Water Contamination
Private Wells Diseases
Heavy Water is a form of water that contains a larger than normal amount of the hydrogen isotope deuterium (2H or D, also known as heavy hydrogen), rather than the common hydrogen-1 isotope (1H or H, also called protium) that makes up most of the hydrogen in normal water. The presence of deuterium gives the chemical different nuclear properties, and the increase of mass gives it different physical and chemical properties compared to normal "light water". (deuterium oxide, 2H2O, D2O).
Hard Water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone and chalk which are largely made up of calcium and magnesium carbonates. Hard drinking water may have moderate health benefits, but can pose critical and grieveous problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling towers, and other equipment that handles water. In domestic settings, hard water is often indicated by a lack of foam formation when soap is agitated in water, and by the formation of limescale in kettles and water heaters. Wherever water hardness is a concern, water softening is commonly used to reduce hard water's adverse effects. Filters.
Soft Water is surface water that contains low concentrations of ions and in particular is low in ions of calcium and magnesium. Soft water naturally occurs where rainfall and the drainage basin of rivers are formed of hard, impervious and calcium poor rocks. Examples in the UK include Snowdonia in Wales and the Western Highlands in Scotland. The term may also be used to describe water that has been produced by a water softening process although such water is more correctly termed softened water. In these cases the water may also contain elevated levels of sodium and/or bicarbonate ions. Because soft water has few calcium ions, there is no inhibition of the lathering action of soaps and no soap scum is formed in normal washing. Similarly, soft water produces no calcium deposits in water heating systems. Water that is not soft is referred to as hard water. In the UK, water is regarded as soft if the hardness is less than 50 mg/l of calcium carbonate. Water containing more than 50 mg/l of calcium carbonate is termed hard water. In the United States soft water is classified as having less than 60 mg/l of calcium carbonate.
Water Softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted mopping up calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins. Borax (wiki).
Limescale is the hard, off-white, chalky deposit found in kettles, hot-water boilers and the inside of inadequately maintained hot-water central heating systems. It is also often found as a similar deposit on the inner surface of old pipes and other surfaces where "hard water" has evaporated.
Saltwater Intrusion is the movement of saline water into freshwater aquifers, which can lead to contamination of drinking water sources and other consequences. Saltwater intrusion occurs naturally to some degree in most coastal aquifers, owing to the hydraulic connection between groundwater and seawater. Because saline water has a higher mineral content than freshwater, it is denser and has a higher water pressure. As a result, saltwater can push inland beneath the freshwater. Certain human activities, especially groundwater pumping from coastal freshwater wells, have increased saltwater intrusion in many coastal areas. Water extraction drops the level of fresh groundwater, reducing its water pressure and allowing saltwater to flow further inland. Other contributors to saltwater intrusion include navigation channels or agricultural and drainage channels, which provide conduits for saltwater to move inland, and it can also make sea level rise. Saltwater intrusion can also be worsened by extreme events like hurricane storm surges.
Hand Pump are manually operated pumps; they use human power and mechanical advantage to move fluids or air from one place to another.
Solar Pump for Water Wells
Heat Tape
Well Pumps
Pressurized Well Tank
Pressure Tank and Well Pump System Basics (youtube)
Water Storage Tanks
Road Salt Pollutes Drinking Water Wells in suburban New York State
Well Drilling is the process of drilling a hole in the ground for the extraction of a natural resource such as ground water.
Deep Hole Drilling is defined as a hole depth greater than ten times the diameter of the hole. These types of holes require special equipment to maintain the straightness and tolerances. Other considerations are roundness and
surface finish. WELL DRILLING 101 | Every Step Explained (youtube).
Drilling Rig is a machine that creates holes in the earth's subsurface. Gun Drilling (wiki).
Borehole is a narrow shaft bored in the ground, either vertically or horizontally. A borehole may be constructed for many different purposes, including the extraction of water. Bore Well is a well of 4 1/2", to 12" in diameter drilled into the earth for retrieving water. A bore well is cased in the region of loose subsoil strata open in hard rock or in crystalline rock. High grade PVC pipes are used for Casing in bore wells. The depth of a bore well can vary from 150 feet to 1500 feet.
Boring into earth is drilling a hole, tunnel, or well in the earth.
Auger Drill is a drilling device, or drill bit, that usually includes a rotating helical screw blade called a "flighting" to act as a screw conveyor to remove the drilled out material. The rotation of the blade causes the material to move out of the hole being drilled.
Rotary Hammer is a power tool that can perform heavy-duty tasks such as drilling and chiseling hard materials.
Dry Land Farming
 Dryland Farming
are agricultural techniques for non-irrigated cultivation of crops.
Dryland farming is associated with drylands - dry areas characterized by a
cool wet season followed by a warm dry season.
Dryland Farming
are agricultural techniques for non-irrigated cultivation of crops.
Dryland farming is associated with drylands - dry areas characterized by a
cool wet season followed by a warm dry season. Agricultural Water Management.
Sustainable Farming - Sustainable Landscaping - Rainwater Management
Desert Farming generally relies on irrigation, as it is the easiest way to make a desert bloom. In California, the Imperial Valley is a good example of what can be done. Australia and the Horn of Africa are also places with interesting desert agriculture.
Desertification is a type of land degradation in which a relatively dry area of land becomes increasingly arid, typically losing its bodies of water as well as vegetation and wildlife. Planting Trees.
Tropical Agriculture common terms would include the humid-tropics (rainforests); the arid-tropics (deserts and dry areas); or monsoon zones (those areas that have well defined wet/dry seasons and experience monsoons). Such labeling is very useful when discussing agriculture, because what works in one area of the world will normally work in a similar area somewhere else, even if that area is on the opposite side of the globe.
International Water Management Institute
Water Management - Irrigation - Droughts - Dormancy
Water User Association is a group of water users, such as irrigators, who pool their financial, technical, material, and human resources for the operation and maintenance of a water system.
Riparian Water Rights is a system for allocating water among those who possess land along its path.
Applied Sciences
EPA Water Conservation Info
Soil Knowledge
North American Water and Power Alliance
Crop Trust
Agricultural Research in the Dry Areas
Food Plants for Dry Regions (PDF)
Simple steps to Climate-Proof Farms have big potential upside for Tropical Farmers. Climate-smart agriculture boosts yields, mitigates extreme weather impact and reduces greenhouse gas emissions. A new study points to profitable opportunities for farmers and the environment.
Heat and Drought-Resistant Tepary Beans are native to the southwestern United States and Mexico and has been grown there by the native peoples since pre-Columbian times. It is more drought-resistant than the common bean (Phaseolus vulgaris) and is grown in desert and semi-desert conditions from Arizona through Mexico to Costa Rica. The water requirements are low and the crop will grow in areas where annual rainfall is less than 400 mm (16 in). 35 Grams Have: Dietary Fiber 15g, Sugars 1g, Protein 8g, Calcium 4%, Iron 8%.
Researchers generate Plants with enhanced Drought Resistance without penalizing growth. A strategy to increase plant hydric stress resistance without affecting overall plant growth.
Tiny plants crucial for sustaining dwindling water supplies. Miniscule plants growing on desert soils can help drylands retain water and reduce erosion, researchers have found. Cyanobacteria in the crusts secrete organic gels and polysaccharides that help to bind small soil particles into stable surfaces. Mosses in the crusts also trapped water and sediment on the soil surface, preventing the removal of soil particles.
Newly Discovered Hormone helps keep Plants from Dehydrating. Peptide CLE25 moves from the roots to the leaves when water is scarce and helps prevent water loss by closing pores in the leaf surface. In animals, peptide hormones are small chains of amino acids that move through the blood and help keep our bodies in balance when the environment changes. For example, when your blood pressure is low, your body produces the hormone vasopressin, which circulates in the blood and acts to narrow your arteries, which increases your blood pressure back to normal levels. Plants also have hormones—called phytohormones—but, scientists know much less about them. The plant scientists at RIKEN CSRS wanted to find out whether any plant hormones respond to physical—abiotic—stress. As first author Fuminori Takahashi explains, “Although we know that some peptide hormones in plants mediate cellular development, until now nobody had identified any that regulate responses to physical stresses such as dehydration.”
UMD Researchers Discover a New Role for a Well-Known Molecule as a Plant Hormone, with Implications for Seed Production and Crop Yield. Plant molecule called ACC or 1-Aminocyclopropane-1-carboxylic acid is a disubstituted cyclic α-amino acid in which a three-membered cyclopropane ring is fused to the Cα atom of the amino acid. ACC plays an important role in the biosynthesis of the plant hormone ethylene.[2 It is synthesized by the enzyme ACC synthase ( EC 4.4.1.14) from methionine and converted to ethylene by ACC oxidase (EC 1.14.17.4).
Gene Regulator that allows Plant Rehydration after Drought. Scientists at the RIKEN Center for Sustainable Resource Science in Japan have found that the protein NGA1 is critical for plants to have normal responses to dehydration. Published in Proceedings of the National Academy of Sciences USA, the study shows how NGA1 controls transcription of a key gene that ultimately allows plants to survive after periods of drought. Plant Breeding.
Scientists Discover how Plants Breathe -- and how humans shaped their 'Lungs'. Experts reveal how plants provide a steady flow of air to every cell. Study shows humans have bred wheat plants to have fewer pores on their leaves and use less water. Findings pave the way to develop more drought-resistant crops.
How Plants Beat the Heat. Researchers at the RIKEN Center for Sustainable Resource Science in Japan have discovered a gene in plants that helps protect them from excessive heat. Published in the scientific journal Plant Cell, the study shows that the newly found gene prevents the destabilization of chloroplast membranes that occurs at very high temperatures.
Scientists engineer crops to conserve water, Resist Drought. Agriculture already monopolizes 90 percent of global freshwater—yet production still needs to dramatically increase to feed and fuel this century’s growing population. For the first time, scientists have improved how a crop uses water by 25 percent without compromising yield by altering the expression of one gene that is found in all plants, as reported in Nature Communications.
Desert “Soilization”: An Eco-Mechanical Solution to Desertification
New technology in China turns desert into land rich with crops (youtube) Paste made from plant cell walls that retains water transforming deserts into forests.
50 Years Ago, This Was a Wasteland. He Changed Everything | Short Film Showcase (youtube) - Planting grasses to soak in rains and fill hillside Aquifers.
Dry Gardening - Landscaping - Farming Knowledge Base
Garden Thorn: water saving technology
Farming without Water - Crop Water Needs
Plants Roots Branch Out to Access Water by sensing the availability of moisture in soil and then adapt their shape to optimise acquisition of water.
Evaporation
Evaporation is a type of vaporization of a liquid that occurs from the surface of a liquid into a gaseous phase that is not saturated with the evaporating substance. The other type of vaporization is boiling, which is characterized by bubbles of saturated vapor forming in the liquid phase. Steam produced in a boiler is another example of evaporation occurring in a saturated vapor phase. Evaporation that occurs directly from the solid phase below the melting point, as commonly observed with ice at or below freezing or moth crystals (napthalene or paradichlorobenzene), is called sublimation, which is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. Evaporation is the process by which water changes from a liquid to a gas or vapor. A substance's state can normally be a solid, liquid, or gas, depending on its temperature and pressure. If the molecules are stuck together really tightly in a regular pattern, then they’re called a solid. The solid form of water is ice. Due to this absorption of energy the hydrogen bonds connecting water molecules to one another will break. The molecules are now in the gaseous state; this is called water vapor. When the molecules are moving fast enough to break the hydrogen bonds, the water can evaporate, turning from liquid to a gas. During evaporation a molecule of water absorbs latent heat. The Hydrogen bonds break. They are intermolecular bonds which exist between molecules, not atoms. The covalent bonds in each water molecule are not affected when water changes state. Hydrogen bonds are weak attractions between Hydrogen atoms on one water molecule and the Oxygen atom on another molecule. Hydrogen bonds can also form between Hydrogen and Nitrogen atoms. The two strands of DNA are held together by Hydrogen bonds. Because the bonds are weaker than covalent bonds, it makes it easy for the DNA to separate into two strands during replication. Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if the water is in ice). When the temperature increases, there are more molecules with higher kinetic energy and thus, more water can evaporate. Water is made up of tiny molecules that are always moving around. The constant movement builds up energy that eventually causes water to evaporate. However, cold water will evaporate much slower than it would if it was hot. When water is hot, the molecules move much faster leading to a quicker evaporation. The pressure due to the ocean of air we live under is about 1 bar or 100 KPascals (units denoting pressure). Liquid water undergoes a phase conversion (called boiling) at 373 K ( = 100 deg C) to steam ( water in the vapor, or gaseous state of matter); this is because the water pressure of the steam equals the pressure of the Earth's atmosphere (mainly containing Nitrogen and Oxygen gases) at 100 deg C. When this happens a tiny gas bubble "nucleases" spontaneously within the liquid water, and the bubble grows and rises in the liquid until it pops out at about 1 bar of water vapor pressure.
Transpiration - Irrigation - Dehydration - Heat - Hot Air - Steam - Boiling
Condensation is the conversion of a vapor or gas into a liquid. The change of the physical state of matter from gas phase into liquid phase, and is the reverse of vapourisation. Water that collects as droplets on a cold surface when humid air is in contact with it. Rain - Water from Air.
Evapotranspiration is the sum of evaporation and plant transpiration from the Earth's land and ocean surface to the atmosphere. Evaporation accounts for the movement of water to the air from sources such as the soil, canopy interception, and waterbodies. Transpiration accounts for the movement of water within a plant and the subsequent loss of water as vapor through stomata in its leaves. Evapotranspiration is an important part of the water cycle. An element (such as a tree) that contributes to evapotranspiration can be called an evapotranspirator.
Cellular Respiration is the process in which nutrients are converted into useful energy in a cell. Photosynthesis.
Aquatic Respiration is when animals are extracting oxygen from water.
Ecosystem Respiration is the measurement of gross carbon dioxide production by all organisms in an ecosystem.
Root Respiration is the exchange of gases between plant roots and the atmosphere.
Respiration in physiology is the transporting oxygen and carbon dioxide between cells and the external environment.
Vapor is a visible suspension in the air of particles of some substance. Boil.
Water Vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Unlike other forms of water, water vapor is invisible. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than air and triggers convection currents that can lead to clouds. Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere where it is also a potent greenhouse gas along with other gases such as carbon dioxide and methane. Use of water vapor, as steam, has been important to humans for cooking and as a major component in energy production and transport systems since the industrial revolution. Water vapor is a relatively common atmospheric constituent, present even in the solar atmosphere as well as every planet in the Solar System and many astronomical objects including natural satellites, comets and even large asteroids. Likewise the detection of extrasolar water vapor would indicate a similar distribution in other planetary systems. Water vapor is significant in that it can be indirect evidence supporting the presence of extraterrestrial liquid water in the case of some planetary mass objects.
Desert Agriculture and Agroforestry
Semiarid Agriculture - Semi-Arid-Climates
Semi-arid Climate is the climate of a region that receives precipitation below potential evapotranspiration, but not extremely. There are different kinds of semi-arid climates, depending on such variables as temperature, and they give rise to different classes of ecology.
Köppen Climate Zone Classification is one of the most widely used climate classification systems. Climate Zones.
Survivopedia
Climate Change - Food Chemistry Dangers
Dry Climate Agriculture - Drought
Desert is a barren area of land where little precipitation occurs and consequently living conditions are hostile for plant and animal life. The lack of vegetation exposes the unprotected surface of the ground to the processes of denudation. About one third of the land surface of the world is arid or semi-arid. This includes much of the polar regions where little precipitation occurs and which are sometimes called polar deserts or "cold deserts". Deserts can be classified by the amount of precipitation that falls, by the temperature that prevails, by the causes of desertification or by their geographical location.
Arid is characterized by a severe lack of available water, to the extent of hindering or preventing the growth and development of plant and animal life. Environments subject to arid climates tend to lack vegetation and are called xeric or desertic. Most "arid" climates surround the equator; these places include most of Africa and parts of South America, Central America and Australia.
Trees - Permaculture
Arable Land is land capable of being ploughed and used to grow crops. In Britain, it was traditionally contrasted with pasturable lands such as heaths which could be used for sheep-rearing but not farmland.
Erosion
Erosion is the action of surface processes (such as water flow, Rain or wind) that remove soil, rock, or dissolved material from one location on the Earth's crust, then transport it away to another location. The particulate breakdown of rock or soil into clastic sediment is referred to as physical or mechanical erosion; this contrasts with chemical erosion, where soil or rock material is removed from an area by its dissolving into a solvent (typically water), followed by the flow away of that solution. Eroded sediment or solutes may be transported just a few millimetres, or for thousands of kilometres. Glaciers - Wind.
Soil Erosion Threats. 6.9 billon tons of soil are eroded each year in America. Soil Expansion - Sinkholes.
Entrainment in physical geography is the process by which surface sediment is incorporated into a fluid flow (such as air, water or even ice) as part of the operation of erosion. Soil Liquefaction.
Solvent is a substance that dissolves a solute or a chemically distinct liquid, solid or gas, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within a cell. Solvents have various applications in chemical syntheses and purification processes. Showering - Washing Hands.
Storm Water Management - Landscaping
How we can make Crops Survive without Water (video and text)
Protecting a Slope from Erosion. Plant grass and shrubs. Bare soil is easily swept away by wind and water, the two main causes of erosion. Add mulch or rocks or spread loose straw and completely cover the exposed soil. Use mulch matting to hold vegetation on slopes. Put down fiber logs. Build retaining walls. Improve drainage. Reduce watering if possible. Avoid soil compaction.
Hydrogeologist – Groundwater Hydrologist specializes in atmospheric and surface/subsurface interactions. Hydrogeologists typically train in departments of geology whereas groundwater hydrologists usually study within engineering departments, although interdisciplinary programs are becoming more common. Hydrogeologists and groundwater hydrologists and engineers evaluate the quantity, quality, reliability, and sustainability of all aspects of groundwater assessment, management, and development.
Hydrology is the scientific study of the movement, distribution, and quality of water on Earth and other planets, including the water cycle, water resources and environmental watershed sustainability. A practitioner of hydrology is a hydrologist, working within the fields of earth or environmental science, physical geography, geology or civil and environmental engineering. Hydrology subdivides into surface water hydrology, groundwater hydrology (hydrogeology), and marine hydrology. Domains of hydrology include hydrometeorology, surface hydrology, hydrogeology, drainage-basin management and water quality, where water plays the central role. Oceanography and meteorology are not included because water is only one of many important aspects within those fields. Hydrological research can inform environmental engineering, policy and planning. .
On-Farm Flood Capture could Reduce Groundwater Overdraft in Kings River Basin
Infiltration in hydrology - Hydraulic Conductivity
Growing Food in Space - Water From Air
Desertification is a type of land degradation in which relatively dry area of land becomes increasingly arid, typically losing its bodies of water as well as vegetation and wildlife.
Orography is the study of the topographic relief of mountains, and can more broadly include hills, and any part of a region's elevated terrain. Orography (also known as oreography, orology or oreology) falls within the broader discipline of geomorphology.
Allan Savory: Make Deserts Green (video)
Green Gold: Reversing Deserts (video)
A forgotten Ancient Grain that could help Africa Prosper (video and interactive text)
Fonio is the term for two cultivated grains in the Digitaria genus which are notable crops in parts of West Africa. The grains are very small. The crops have C4 metabolisms and are medium in height. The number of chromosomes for the species can be diploid (2n), tetraploid (4n), or hexaploid (6n). also called "hungry rice," is the most important of a diverse group of wild and domesticated Digitaria species that are harvested in the savannas of West Africa. Fonio has the smallest seeds of all species of millet. It has potential to improve nutrition, boost food security, foster rural development, and support sustainable use of the land.Fonio has continued to be important locally because it is both nutritious and one of the world's fastest-growing cereals, reaching maturity in as little as six to eight weeks. It is a crop that can be relied on in semi-arid areas with poor soils, where rains are brief and unreliable. The grains are used in porridge and couscous, for bread, and for beer. The small grains make it difficult and time-consuming to remove the husk. Traditional methods include pounding it in a mortar with sand (then separating the grains and sand) or "popping" it over a flame and then pounding it (which yields a toasted-color grain; this technique is used among the Akposso). The invention of a simple fonio husking machine offers an easier mechanical way to dehusk.
A Cereal Survives Heat and Drought. Pearl millet genome sequence provides a resource to improve agronomic traits in extreme environments. Pearl millet is the most widely grown type of millet. It has been grown in Africa and the Indian subcontinent since prehistoric times. Millet are a group of highly variable small-seeded grasses, widely grown around the world as cereal crops or grains for fodder and human food. Millets are important crops in the semiarid tropics of Asia and Africa (especially in India, Mali, Nigeria, and Niger), with 97% of millet production in developing countries. The crop is favored due to its productivity and short growing season under dry, high-temperature conditions.
Savory Institute
What if we Change
Africa Centre For Holistic Management
Holistic Management
Earthworks
Holistic Management is a systems thinking approach to managing resources that was originally developed by Allan Savory for reversing desertification.
Rangeland are grasslands, shrublands, woodlands, wetlands, and deserts that are grazed by domestic livestock or wild animals. Types of rangelands include tallgrass and shortgrass prairies, desert grasslands and shrublands, woodlands, savannas, chaparrals, steppes, and tundras. Rangelands do not include forests lacking grazable understory vegetation, barren desert, farmland, or land covered by solid rock, concrete and/or glaciers.
Grazing is a method of feeding in which a herbivore feeds on plants such as grasses, or other multicellular organisms such as algae. In agriculture, grazing is one method used whereby domestic livestock are used to convert grass and other forage into meat, milk and other products.
Ecology - Environmental Awareness
Natural Sequence Farming is a method of landscape regeneration that involves implementing major earthworks on a given area of land that has been devastated by deforestation and general agricultural activities, to emulate the role of natural watercourses in an effort to reverse salinity, slow erosion and increase soil and water quality to enable native vegetation to regenerate and restore the riparian zone. The method does not require the use of artificial fertilizers or herbicides. Natural Sequence Farming - Natural Sequence Farming.
Drip Irrigation
Drip Irrigation is a form of irrigation that saves water and fertilizer by allowing water to drip slowly to the roots of many different plants, either onto the soil surface or directly onto the root zone, through a network of valves, pipes, tubing, and emitters. It is done through narrow tubes that deliver water directly to the base of the plant. It is chosen instead of surface irrigation for various reasons, often including concern about minimizing evaporation.
Drip Irrigation Tools - Irrigation water use - Water for Farming Use
Irrigation is the method in which a controlled amount of water is supplied to plants at regular intervals for agriculture. It is used to assist in the growing of agricultural crops, maintenance of landscapes, and revegetation of disturbed soils in dry areas and during periods of inadequate rainfall. Additionally, irrigation also has a few other uses in crop production, which include protecting plants against frost, suppressing weed growth in grain fields and preventing soil consolidation. In contrast, agriculture that relies only on direct rainfall is referred to as rain-fed or dry land farming. Irrigation systems are also used for dust suppression, disposal of sewage, and in mining. Irrigation is often studied together with drainage, which is the natural or artificial removal of surface and sub-surface water from a given area.
Irrigation - Soil - Erosion - Dry Land Farming
Center Pivot Irrigation is a method of crop irrigation in which equipment rotates around a pivot and crops are watered with sprinklers. A circular area centered on the pivot is irrigated, often creating a circular pattern in crops when viewed from above (sometimes referred to as crop circles). Most center pivots were initially water-powered, and today most are propelled by electric motors. Irrigation For Farming Could Leave Many Of The World's Streams And Rivers Dry.
Infiltration in hydrology is the process by which water on the ground surface enters the soil. Infiltration rate in soil science is a measure of the rate at which soil is able to absorb rainfall or irrigation. It is measured in inches per hour or millimeters per hour. The rate decreases as the soil becomes saturated. If the precipitation rate exceeds the infiltration rate, runoff will usually occur unless there is some physical barrier. It is related to the saturated hydraulic conductivity of the near-surface soil. The rate of infiltration can be measured using an infiltrometer, which is a device used to measure the rate of water infiltration into soil or other porous media. Commonly used infiltrometers are single ring or double ring infiltrometer, and also disc permeameter, which is a field instrument used for measuring water infiltration in the soil, which is characterized by in situ saturated and unsaturated soil hydraulic properties. It is mainly used to provide estimates of the hydraulic conductivity of the soil near saturation.
Hydraulic Conductivity is a property of vascular plants, soils and rocks, that describes the ease with which a fluid (usually water) can move through pore spaces or fractures. It depends on the intrinsic permeability of the material, the degree of saturation, and on the density and viscosity of the fluid. Saturated hydraulic conductivity, Ksat, describes water movement through saturated media. By definition, hydraulic conductivity is the ratio of velocity to hydraulic gradient indicating permeability of porous media.
Farm Water is water committed for use in the production of food and fiber. On average, 80 percent of the fresh water withdrawn from rivers and groundwater is used to produce food and other agricultural products. Farm water may include water used in the irrigation of crops or the watering of livestock. Water Use Stats
Swale Landform is a low tract of land, especially one that is moist or marshy. The term can refer to a natural landscape feature or a human-created one. Artificial swales are often designed to manage water runoff, filter pollutants, and increase rainwater infiltration.
More-Crop-Per-Drop - Micro-Sprinklers low-pressure bayonet.
Zai is a farming technique to dig pits (20-30 cm long and deep and 90 cm apart) in the soil during the preseason to catch water and concentrate compost. The technique is traditionally used in western Sahel (Burkina Faso, Niger, Mali) to restore degraded drylands and increase soil fertility. Zaï holes were reintroduced since the 1980s by Yacouba Sawadogo, a farmer from Burkina Faso, who introduced the innovation of filling them with manure and compost to provide plant nutrients. The manure attracts termites, whose tunnels help further break up the soil. He also slightly increased the size of the holes over the traditional models. Zaï holes help by improving the yields of trees, sorghum, and millet by up to 500 percent. As an alternative to the zaï-technique some agricultural engineers suggest a diking technique, especially in the case of very light soils.(tassa irrigation method).
Gravity Feed is the use of earth's gravity to move something (usually a liquid) from one place to another. It is a simple means of moving a liquid without the use of a pump. A common application is the supply of fuel to an internal combustion engine by placing the fuel tank above the engine, e.g. in motorcycles, lawn mowers, etc. A non-liquid application is the carton flow shelving system.
Culvert is a structure that allows water to flow under a road, railroad, trail, or similar obstruction from one side to the other. Typically embedded so as to be surrounded by soil, a culvert may be made from a pipe, reinforced concrete or other material. In the United Kingdom, the word can also be used for a longer artificially buried watercourse. Culverts are commonly used both as cross-drains to relieve drainage of ditches at the roadside, and to pass water under a road at natural drainage and stream crossings. A culvert may be a bridge-like structure designed to allow vehicle or pedestrian traffic to cross over the waterway while allowing adequate passage for the water. Culverts come in many sizes and shapes including round, elliptical, flat-bottomed, open-bottomed, pear-shaped, and box-like constructions. The culvert type and shape selection is based on a number of factors including requirements for hydraulic performance, limitations on upstream water surface elevation, and roadway embankment height. The process of removing culverts to restore an open-air watercourse is known as daylighting. In the UK, the practice is also known as deculverting.
Sustainable Landscaping (lawns)
Rainbird - Rain Garden
Irritrol PC Control
Irrigation Management Systems
Water Professionals
Agricultural Dewatering
Rob Harmon: How to keep streams flowing (youtube)
Water Blueprint
North of the 49th parallel of latitude. Productive farming, therefore, depends on crops that ripen early, if they are spring sown, or are winter hardy, if they are winter annuals, biennials or perennials. Crops can be classified in several ways. By growth habit they are annual, biennial or perennial, depending on whether they complete their life cycle in one or two years, or persist for over two years. The special term "winter annuals" is used for crops that are planted and germinate in fall, spend winter in a dormant state, renew growth in spring and are harvested in July or August.
Climate Categories in Viticulture are categorised based on the overall characteristics of the area's climate during the growing season.
Subtropics are geographic and climate zones located roughly between the tropics at latitude 23.5° (the Tropic of Cancer and Tropic of Capricorn) and temperate zones (normally referring to latitudes 35–66.5°) north and south of the Equator.
Tropical Agriculture common terms would include the humid-tropics (rainforests); the arid-tropics (deserts and dry areas); or monsoon zones (those areas that have well defined wet/dry seasons and experience monsoons). Such labeling is very useful when discussing agriculture, because what works in one area of the world will normally work in a similar area somewhere else, even if that area is on the opposite side of the globe. Most temperate zone agricultural techniques are inappropriate for tropical areas.
An Engineer Created Growable Ice Towers to help combat Drought.
Ice Spike is an ice formation, often in the shape of an inverted icicle, that projects upwards from the surface of a body of frozen water. Ice spikes created by natural processes on the surface of small bodies of frozen water have been reported for many decades, although their occurrence is quite rare.
Drought - No Rain - No More Glaciers
Drought is a period of below-average precipitation in a given region, resulting in prolonged shortages in its water supply, whether atmospheric, surface water or ground water. A drought can last for months or years, or may be declared after as few as 15 days. It can have a substantial impact on the ecosystem and agriculture of the affected region and harm to the local economy. Annual dry seasons in the tropics significantly increase the chances of a drought developing and subsequent bush fires. Periods of heat can significantly worsen drought conditions by hastening evaporation of water vapour.
Drought Monitor - The National Drought Mitigation Center
Climate-Related Threats to Global Food Production include risks to grain, vegetable, and fruit crops, livestock, and fisheries.
Climate change and Food Security - Food Security
Faster, more Accurate way to Monitor Drought. By combining surface and air temperature measurements from thousands of weather stations and satellite images, we can monitor current conditions across an entire region in near real time and identify the specific places where drought-induced thermal stress is occurring. Drought Eye.
Water Risk Areas - Hibernation (drought resistance)
Global Precipitation Measurements - Dry Land Farming
Field of Vision - Scenes from a Dry City. What happens when a major metropolitan area runs out of water? In Cape Town, South Africa, residents fear the arrival of “Day Zero,” when the city’s taps will be shut off.
Climate-driven megadrought is emerging in western US, says study.
United Nations Environment Programme (UNEP) says that crops such as wheat and maize are generating more potential toxins as a reaction to protect themselves from extreme weather. These chemical compounds are harmful to people and animals if consumed for a prolonged period of time. Under normal conditions, for instance, plants convert nitrates they absorb into nutritious amino acids and proteins. But prolonged drought slows or prevents this conversion, leading to more potentially problematic nitrate accumulating in the plant. If people eat too much nitrate in their diets, it can interfere with the ability of red blood cells to transport oxygen in the body, Crops susceptible to accumulating too much nitrate in times of stress include maize, wheat, barley, soybeans, millet and sorghum. Some drought-stressed crops, when then exposed to sudden large amounts of rain that lead to rapid growth, in turn accumulate hydrogen cyanide, more commonly known as prussic acid. Prussic acid - one of the ingredients used in some types of chemical warfare - interferes with oxygen flow in humans. Plants such as cassava, flax, maize and sorghum are most vulnerable to dangerous prussic acid accumulation. Cases of nitrate or hydrogen cyanide poisoning in humans were reported in Kenya in 2013 and in the Philippines in 2005. Aflatoxins, molds that can affect plant crops and raise the risk of liver damage, cancer and blindness, as well as stunting foetuses and infants. About 4.5 billion people in developing countries are exposed to aflatoxins each year Toxic crops can lead to neurological diseases among humans but the greatest challenge is the incidence of cancer. Research centers with the Consultative Group on International Agricultural Research are developing seeds that are suitable in various regions that have been hit by climate change.
1.5 billion people will depend on water from mountains. Global water consumption has increased almost fourfold in the past 100 years, and many regions can only meet their water demand thanks to essential contributions from mountain regions. In 30 years, almost a quarter of the world's lowland population will strongly depend on runoff from the mountains. Only sustainable development can ensure the important function of mountain areas as Earth's ''water towers''.
Rain - Precipitation - Water Cycle
Rain is liquid water in the form of droplets or really small round sphericals of water that have condensed from atmospheric water vapor and then precipitated and become heavy enough to fall under gravity. Raindrops begin forming when water vapor condenses on micrometer-sized particles of dust floating in the atmosphere. (pressure, temperature and electrical charge). The dust particles grow to millimeter-sized droplets, which are heavy enough to begin falling. Raindrops form in a roughly spherical structure due to the surface tension of water. The surface tension is stronger on smaller raindrops than in larger drops. Rain is a major component of the water cycle and is responsible for depositing most of the fresh water on the Earth. Rain provides suitable conditions for many types of ecosystems, as well as water for hydroelectric power plants and crop irrigation.
Weather - Volunteers Measure Precipitation from each Nation.
Precipitation is the quantity of water falling to earth at a specific place within a specified period of time. Any form of water like rain, snow, hail, sleet or mist that falls to earth, ground or sea. Precipitate is a suspension of newly formed particles falling from the clouds, like rain drops, snow flakes, hail stones, sleet or mist.
Water Cycle describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly constant over time but the partitioning of the water into the major reservoirs of ice, fresh water, saline water and atmospheric water is variable depending on a wide range of climatic variables. The water moves from one reservoir to another, such as from river to ocean, or from the ocean to the atmosphere, by the physical processes of evaporation, condensation, precipitation, infiltration, surface runoff, and subsurface flow. In doing so, the water goes through different forms: liquid, solid (ice) and vapor. The water cycle involves the exchange of energy, which leads to temperature changes. When water evaporates, it takes up energy from its surroundings and cools the environment. When it condenses, it releases energy and warms the environment. These heat exchanges influence climate. The evaporative phase of the cycle purifies water which then replenishes the land with freshwater. The flow of liquid water and ice transports minerals across the globe. It is also involved in reshaping the geological features of the Earth, through processes including erosion and sedimentation. The water cycle is also essential for the maintenance of most life and ecosystems on the planet. (also known as the hydrologic cycle or the hydrological cycle).
Surface Runoff is the flow of water that occurs when excess storm water, meltwater, or other sources flows over the Earth's surface. This might occur because soil is saturated to full capacity, because rain arrives more quickly than soil can absorb it, or because impervious areas (roofs and pavement) send their runoff to surrounding soil that cannot absorb all of it. Surface runoff is a major component of the water cycle. It is the primary agent in soil Erosion by water.
Urban Runoff is surface runoff of rainwater created by urbanization. This runoff is a major source of flooding and water pollution in urban communities worldwide. Impervious surfaces (roads, parking lots and sidewalks) are constructed during land development. During rain storms and other precipitation events, these surfaces (built from materials such as asphalt and concrete), along with rooftops, carry polluted stormwater to storm drains, instead of allowing the water to percolate through soil. This causes lowering of the water table (because groundwater recharge is lessened) and flooding since the amount of water that remains on the surface is greater. Most municipal storm sewer systems discharge stormwater, untreated, to streams, rivers and bays. This excess water can also make its way into people's properties through basement backups and seepage through building wall and floors.
Storm Water Management is the quantity and quality of stormwater manage using Best Management Practice (BMP) or stormwater control measure (SCM). Often used to refer to both structural or engineered control devices and systems (e.g. retention ponds) to treat or store polluted stormwater, as well as operational or procedural practices (e.g. street sweeping). Stormwater management includes both technical and institutional aspects, including: Control of flooding and erosion; Control of hazardous materials to prevent release of pollutants into the environment (source control); Planning and construction of stormwater systems so contaminants are removed before they pollute surface waters or groundwater resources; Acquisition and protection of natural waterways or rehabilitation; Building "soft" structures such as ponds, swales, wetlands or green infrastructure solutions to work with existing or "hard" drainage structures, such as pipes and concrete channels; Development of funding approaches to stormwater programs potentially including stormwater user fees and the creation of a stormwater utility; Development of long-term asset management programs to repair and replace aging infrastructure; Revision of current stormwater regulations to address comprehensive stormwater needs; Enhancement and enforcement of existing ordinances to make sure property owners consider the effects of stormwater before, during and after development of their land; Education of a community about how its actions affect water quality, and about what it can do to improve water quality; and planning carefully to create solutions before problems become too great. EPA Facility Stormwater Management.
Drainage is the natural or artificial removal of a surface's water and sub-surface water from an area with excess of water. The internal drainage of most agricultural soils is good enough to prevent severe waterlogging (anaerobic conditions that harm root growth), but many soils need artificial drainage to improve production or to manage water supplies.
Return Flow is surface and subsurface water that leaves the field following application of irrigation water. While irrigation return flows is a point source, they are expressly exempted from permit requirements under the Clean Water Act (P.L. 92-500, as amended). Return flows generally return to the irrigation centre after a period of about three to four weeks; due to this, the farmers usually need to pour bleach into the water to clean it of any organisms that have entered the stream. If this is not taken care of, diseases such as typhoid or cholera could enter the irrigation and pose a risk of epidemic disease to surrounding towns and cities.
Bioretention is the process in which contaminants and sedimentation are removed from stormwater runoff. Stormwater is collected into the treatment area which consists of a grass buffer strip, sand bed, ponding area, organic layer or mulch layer, planting soil, and plants. Runoff passes first over or through a sand bed, which slows the runoff's velocity, distributes it evenly along the length of the ponding area, which consists of a surface organic layer and/or groundcover and the underlying planting soil. The ponding area is graded, its center depressed. Water is ponded to a depth of 15 cm (5.9 in) and gradually infiltrates the bioretention area or is evapotranspired. The bioretention area is graded to divert excess runoff away from itself. Stored water in the bioretention area planting soil exfiltrates over a period of days into the underlying soils.
Groundwater Recharge is a hydrologic process, where water moves downward from surface water to groundwater. Recharge is the primary method through which water enters an aquifer. This process usually occurs in the vadose zone below plant roots and, is often expressed as a flux to the water table surface. Groundwater recharge also encompasses water moving away from the water table farther into the saturated zone. Recharge occurs both naturally (through the water cycle) and through anthropogenic processes (i.e., "artificial groundwater recharge"), where rainwater and or reclaimed water is routed to the subsurface.
Topmix Permeable is a fast draining concrete pavement solution that rapidly directs stormwater off streets, parking surfaces, driveways and walkways. This minimises the cost and long-term maintenance for local authorities and developers of stormwater management.
Low-Impact Development describes a land planning, and engineering design approach to manage stormwater runoff as part of green infrastructure. LID emphasizes conservation and use of on-site natural features to protect water quality. This approach implements engineered small-scale hydrologic controls to replicate the pre-development hydrologic regime of watersheds through infiltrating, filtering, storing, evaporating, and detaining runoff close to its source. ’Green infrastructure’ investments are one approach that often yields multiple benefits and builds city resilience.
Floodplain is an area of land adjacent to a stream or river which stretches from the banks of its channel to the base of the enclosing valley walls and which experiences flooding during periods of high discharge. The soils usually consist of levees, silts, and sands deposited during floods. Levees are the heaviest materials (usually pebble-size) and they are deposited first; silts and sands are finer materials.
Retention Basin is used to manage stormwater runoff to prevent flooding and downstream erosion, and improve water quality in an adjacent river, stream, lake or bay. Sometimes called a wet pond or wet detention basin or stormwater management pond, it is an artificial lake with vegetation around the perimeter, and includes a permanent pool of water in its design.
Detention Basin is an excavated area installed on, or adjacent to, tributaries of rivers, streams, lakes or bays to protect against flooding and, in some cases, downstream erosion by storing water for a limited period of time. A detention basin, sometimes called a "dry pond", which temporarily stores water after a storm, but eventually empties out at a controlled rate to a downstream water body. It also differs from an infiltration basin which is designed to direct stormwater to groundwater through permeable soils.
Rain Catchers (saving some of the rain to use at a later time)
Rain Harvesting Barrels (amazon) - Rain Barrel - Rainwater Harvesting
Saving Rain Tools
Snowpack forms from layers of snow that accumulate in geographic regions and high altitudes where the climate includes cold weather for extended periods during the year. Snowpacks are an important water resource that feed streams and rivers as they melt. Therefore, snowpacks are both the drinking water source for many communities and a potential source of flooding (in case of sudden melting). Snowpacks also contribute mass to glaciers in their accumulation zone.
Snowmelt is surface runoff produced from melting snow. It can also be used to describe the period or season during which such runoff is produced. Water produced by snowmelt is an important part of the annual water cycle in many parts of the world, in some cases contributing high fractions of the annual runoff in a watershed. Predicting snowmelt runoff from a drainage basin may be a part of designing water control projects. Rapid snowmelt can cause flooding. If the snowmelt is then frozen, very dangerous conditions and accidents can occur, introducing the need for salt to melt the Ice.
Meltwater is water released by the melting of snow or ice, including glacial ice, tabular icebergs and ice shelves over oceans. Meltwater is often found in the ablation zone of glaciers, where the rate of snow cover is reducing. Meltwater can be produced during volcanic eruptions, in a similar way in which the more dangerous lahars form.
Dust speeds up spring water runoff causing intense melting and streams to peak weeks earlier than usual — which wreaks havoc throughout the alpine ecosystem. Without dirt or dust, the snow melts off slow and steady. Most of the dust that's settling in places like the San Juan mountains comes from the desert southwest, from land disturbances like farming, oil and gas drilling, cattle grazing, recreation and residential development on the southern end of the Colorado Plateau.
Radiative Forcing is the difference between insolation (sunlight) absorbed by the Earth and energy radiated back to space. The influences that cause changes to the Earth’s climate system altering Earth’s radiative equilibrium, forcing temperatures to rise or fall, are called climate Forcings. Positive radiative forcing means Earth receives more incoming energy from sunlight than it radiates to space. This net gain of energy will cause warming. Conversely, negative radiative forcing means that Earth loses more energy to space than it receives from the sun, which produces cooling. Dust Radiative Forcing.
Blood Rain is a phenomenon in which blood is perceived to fall from the sky in the form of rain.
Rivers
Haematococcus Pluvialis is a freshwater species of Chlorophyta from the family Haematococcaceae. This species is well known for its high content of the strong antioxidant astaxanthin, which is important in aquaculture, and cosmetics. Often responsible for the blood-red colour seen in the bottom of dried out rock pools and bird baths.
How Much Water Do I Use?
'You can’t manage what you don’t measure.'
Water Management is the activity of planning, developing, distributing and managing the optimum use of water resources. It is a sub-set of water cycle management. Ideally, water resource management planning has regard to all the competing demands for water and seeks to allocate water on an equitable basis to satisfy all uses and demands. As with other resource management, this is rarely possible in practice.
Water Conservation - Tools
Water Sense (EPA)
Water Use Today (EPA)
Water Home Use
Water Use by State Info-Graph (image)
Average Water Use
Water Consumption Calculator
Water Use it Wisely
Watershed Management is the study of the relevant characteristics of a watershed aimed at the sustainable distribution of its resources and the process of creating and implementing plans, programs, and projects to sustain and enhance watershed functions that affect the plant, animal, and human communities within a watershed boundary. Features of a watershed that agencies seek to manage include water supply, water quality, drainage, stormwater runoff, water rights, and the overall planning and utilization of watersheds. Landowners, land use agencies, storm water management experts, environmental specialists, water use surveyors and communities all play an integral part in watershed management.
Watershed is a ridge of land that separates two adjacent river systems. The entire geographical area drained by a river and its tributaries; an area characterized by all runoff being conveyed to the same outlet. Rain.
Virtual Water refers to the hidden flow of water if food or other commodities are traded from one place to another. For instance, it takes 1,340 cubic meters of water (based on the world average) to produce one metric tonne of wheat. The precise volume can be more or less depending on climatic conditions and agricultural practice. Hoekstra and Chapagain have defined the virtual-water content of a product (a commodity, good or service) as "the volume of freshwater used to produce the product, measured at the place where the product was actually produced". It refers to the sum of the water use in the various steps of the production chain.
Water Footprint shows the extent of water use in relation to consumption of people. The water footprint of an individual, community or business is defined as the total volume of fresh water used to produce the goods and services consumed by the individual or community or produced by the business. Water use is measured in water volume consumed (evaporated) and/or polluted per unit of time. A water footprint can be calculated for any well-defined group of consumers (e.g., an individual, family, village, city, province, state or nation) or producers (e.g., a public organization, private enterprise or economic sector), for a single process (such as growing rice) or for any product or service. Traditionally, water use has been approached from the production side, by quantifying the following three columns of water use: water withdrawals in the domestic, agricultural and industrial sector. While this does provide valuable data, it is a limited way of looking at water use in a globalised world, in which products are not always consumed in their country of origin. International trade of agricultural and industrial products in effect creates a global flow of virtual water, or embodied water (akin to the concept of embodied energy). In 2002, the water footprint concept was introduced in order to have a consumption-based indicator of water use, that could provide useful information in addition to the traditional production-sector-based indicators of water use. It is analogous to the ecological footprint concept introduced in the 1990s. The water footprint is a geographically explicit indicator, not only showing volumes of water use and pollution, but also the locations. Thus, it gives a grasp on how economic choices and processes influence the availability of adequate water resources and other ecological realities across the globe (and vice versa).
80 percent of the Fresh Water withdrawn from rivers and groundwater is used to produce food and other agricultural products.
Water for Farming Use - Irrigation - Vertical Farming uses 90% less water.
Did you know that it takes almost 2,000 gallons of water to produce 1 pound of meat?
It takes around 1 gallon of water to produce one almond.
It takes almost 25 gallons of water to produce 1 pound of wheat.
It takes around 16 gallons water to make a single cup of beer.
Did you know that a Mediterranean Diet causes less pollution?
How much water is needed to make your Food (calculator)?
8-minute shower runs through about 20 gallons of water on average.
Nebia Shower Head uses 70% Less Water
Teaching people about cause and effect is extremely important.
Nearly one person in six lives without regular access to safe drinking water, and more than twice that many lack access to Adequate Sanitation. Water-related diseases kill a child every eight seconds, and are responsible for 80 percent of all easily preventable illnesses and deaths in the developing world.
Droppler: Know your Habits. Save Water
"Things that are widely used by many life forms can have an impact to the health of our earth and it's inhabitants. Please don't hurt the water, it has given us life for millions of years."
Drought Shame App - Drought Information
Power Plants draw more Fresh Water than any other consumer in the United States, accounting for more than 50 percent of the nation's freshwater use at about 500 billion gallons daily. To help save this water, researchers have developed a new silica filter for power plant cooling waters that decreases the amount of freshwater power plants consume by increasing the number of times cooling tower water can be reused and recycled.
Recycling Water - Water Saving Tools
Living Machines form of ecological sewage treatment designed to mimic the cleansing functions of wetlands.
Living Machines - Living Machine
Todd Ecological - Bio-Mimicry
Grey Water People
Flotender
Grey Water Action
Grey Water Sustainable Sources
Biopore Holes not only helps to absorb rainwater, but also functions as a waste management system for organic waste.
Water Tanks
Water Barrels
Rain Water Collection (Tank Town)
Rain Scaping
Rain Catchers
Rain Harvesting Barrels (amazon)
Rain Barrel
Water Storage Tanks
Water Storage Tanks
Water Tank is a container for storing water. The need for a water tank is as old as civilization, to provide storage of water for use in many applications, drinking water, irrigation agriculture, fire suppression, agricultural farming, both for plants and livestock, chemical manufacturing, food preparation as well as many other uses. Water tank parameters include the general design of the tank, and choice of construction materials, linings. Various materials are used for making a water tank: plastics (polyethylene, polypropylene), fiberglass, concrete, stone, steel (welded or bolted, carbon, or stainless). Earthen pots also function as water storages. Water tanks are an efficient way to help developing countries to store clean water.
Wells
Groasis Aquapro Water Box
Hippo Roller - 15 Methods for Transporting Water
Rain Reserve - Enviro Sink - Rain (knowledge)
Transporting Water : Water Transportation is the intentional movement by water over large distances. Methods of transportation fall into three categories: Aqueducts, which include pipelines, canals, and tunnels, container shipment, which includes transport by tank truck, tank car, and tank ship, and towing, where a tugboat is used to pull an iceberg or a large water bag along behind it.
Water Conservation
Water Conservation includes all the policies, strategies and activities made to sustainably manage the natural resource fresh water, to protect the water environment, and to meet current and future human demand. Population, household size, and growth and affluence all affect how much water is used. Factors such as climate change have increased pressures on natural water resources especially in manufacturing and agricultural irrigation. Many US cities have already implemented policies aimed at water conservation, with much success. The goals of water conservation efforts include: Ensuring availability of water for future generations where the withdrawal of freshwater from an ecosystem does not exceed its natural replacement rate. Energy conservation as water pumping, delivery and wastewater treatment facilities consume a significant amount of energy. In some regions of the world over 15% of total electricity consumption is devoted to water management. Habitat conservation where minimizing human water use helps to preserve freshwater habitats for local wildlife and migrating waterfowl, but also water quality. The key activities that benefit water conservation are as follows: Any beneficial reduction in water loss, use and waste of resources. Avoiding any damage to water quality. Improving water management practices that reduce the use or enhance the beneficial use of water.
Water Conserve - Tips - Soft Water Path
Measuring Water Usage - Saving Water Tools
Water Saving Toilets - Toilets (types) - Toilet Condensation or toilet sweats.
Meet Flo is a comprehensive water monitoring and shut-off system with leak detection and proactive leak prevention technologies. Via its smartphone dashboard, Flo by Moen provides the ability to monitor and control your water remotely, while empowering conservation efforts. See how much water you’re consuming with daily trends.
Showers that save water
Recycling Shower
Eva Smart Shower
1.6 GPM Showerhead (amazon)
Nebia Shower System
Cirrus Shower uses 75 percent less water.
Altered Nozzle changes your existing faucet saving 98% water.
Drybath helps keep the body clean without water. Hygiene
Water 2 Save water management service.
Water Saving Tools for home and yard.
Water Saver Technologies
Niagara Conservation
Conserv Co
Dry Planet
Slow Growing Grass needs less water
Energy Saving Tools
Ancient intervention could boost dwindling water reserves in coastal Peru. Ancient Peruvian civilizations in 600 AD created systems within mountains to divert excess rainwater from source streams onto mountain slopes and through rocks. The water would take some months to trickle through the system and resurface downstream -- just in time for the dry season
Swimming Pools
Ionized Swimming Pool - Chlorine Free Swimming Pools.
Chemical Free Swimming Pools - Very Low Energy Consumption & Healthy.
Aqua-Scapes Pool - Biological and Natural Swimming Pools using no chemicals.
Hidden Dual Purpose Pools
Swim in Place Pool
Swimming Machine is a resistance swimming apparatus, often self-contained, enabling the swimmer to swim in place. This may be accomplished either by accelerating the water past the swimmer or by supporting the swimmer, either in water or on dry land.
Swimex
Swimming Knowledge
Nemo 33 in Uccle, Belgium is the deepest indoor swimming pool in the world.
World's Largest Outdoor Pool At Chile's San Alfonso del Mar Resort.
Flowavett Wave Pool
Delta Flume wave generator that is capable of producing waves as tall as five meters the world's largest artificial waves.
In one of every eight pools inspected, the violations were so serious the pools were forced to close immediately. Improperly maintained pools have been linked to a variety of accidents and illnesses, from drowning to intestinal parasites, the CDC said. About 4,000 people drown in pools and elsewhere each year in the United States, and there were nearly 350 disease outbreaks linked to pools from 2003 to 2012.
Automatic Watering Systems
CONTINENTAL AWS-10 Automatic Watering System for containers (amazon)
Single-Dial Water Timer (amazon)
Drip Irrigation Spikes (amazon) - Watering Spikes (amazon)
Tree I.V.® Portable Watering System 6-pack (amazon)
How to install an automated drip irrigation system video with Thompson & Morgan (youtube)
Automatic Drip Irrigation Kit
Potted Plants Watering System Kit. Automatic drip irrigation system for large size ( 10-inch pots or bigger) potted plants. Waters directly into plant roots avoiding evaporation Equipped with 4 color-coded drippers with daily water release: 3.4 ounce-20 days, 5.1 ounce-13 days, 6.8 ounce-10 days, 10.1 ounce-6 days.
Water Knowledge - Basic Facts about Water
H2O - There are 3 Atoms in a water molecule, 2 hydrogen atoms (H), and 1 oxygen atom (O). Dihydrogen monoxide is a name for the water molecule, also known as dihydrogen oxide, hydroxyl acid or hydroxylic acid. Water is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, nearly colorless with a hint of blue. This simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the "universal solvent" for its ability to dissolve many substances. This allows it to be the "solvent of life": indeed, water as found in nature almost always includes various dissolved substances, and special steps are required to obtain chemically pure water. Water is the only common substance to exist as a solid, liquid, and gas in normal terrestrial conditions. Along with oxidane, water is one of the two official names for the chemical compound H2O; it is also the liquid phase of H2O. The other two common states of matter of water are the solid phase, ice, and the gaseous phase, water vapor or steam. The addition or removal of heat can cause phase transitions: freezing (water to ice), melting (ice to water), vaporization (water to vapor), condensation (vapor to water), sublimation (ice to vapor) and deposition (vapor to ice). Because of its polarity, a molecule of water in the liquid or solid state can form up to four hydrogen bonds with neighboring molecules. Hydrogen bonds are about ten times as strong as the Van der Waals force that attracts molecules to each other in most liquids. This is the reason why the melting and boiling points of water are much higher than those of other analogous compounds like hydrogen sulfide. They also explain its exceptionally high specific heat capacity (about 4.2 J/g/K), heat of fusion (about 333 J/g), heat of vaporization (2257 J/g), and thermal conductivity (between 0.561 and 0.679 W/m/K). These properties make water more effective at moderating Earth's climate, by storing heat and transporting it between the oceans and the atmosphere. The hydrogen bonds of water are around 23 kJ/mol (compared to a covalent O-H bond at 492 kJ/mol). Of this, it is estimated that 90% is attributable to electrostatics, while the remaining 10% is partially covalent. These bonds are the cause of water's high surface tension and capillary forces. The capillary action refers to the tendency of water to move up a narrow tube against the force of gravity. This property is relied upon by all vascular plants, such as trees. The diameter of a water molecule is closely calculated to be about 0.000282 µm (micrometers – millionths of a meter) in diameter. Water splits into Hydrogen Ions (H+) and Hydroxyl Ions (OH-). When there are equal parts of Hydrogen Ions (H+) and Hydroxyl Ions (OH-) leading to a 1:1 ratio, pH is neutral (7). When they dissociate to form ions, water molecules therefore form a positively charged H+ ion and a negatively charged OH- ion. The fact that water molecules dissociate to form H+ and OH- ions, which can then recombine to form water molecules. An individual molecule of H2O doesn’t have any of the observable properties we associate with water. A glass of water, pure as water can be, is better understood as containing H2O, OH–, H3O+ and other related but less common ions, and even this is a vast oversimplification (if we could get truly pure water, which we cannot). Our current best understanding of the electron transfers that give water the properties we observe is a statistical average of ever changing interactions so complex as to be quite literally unthinkable. Indeed, the problem is “not that we are unsure which (distribution of types of) microstructure is the correct one. The point is that there is no one correct microstructure, because the microstructure depends as much on the context and functions just as another nominal essence would. To produce two molecules of water (H2O), two molecules of diatomic hydrogen (H2) must be combined with one molecule of diatomic oxygen (O2). Energy will be released in the process. 2H2 + O2 = 2H2O + Energy.
Earth was born with water. Water is Life - Water is Sacred.
Hydrosphere is the combined mass of water found on, under, and above the surface of a planet, minor planet or natural satellite. It has been estimated that there are 1386 million cubic kilometers of water on Earth. This includes water in liquid and frozen forms in groundwater, oceans, lakes and streams. Saltwater accounts for 97.5% of this amount. Fresh water accounts for only 2.5%. Of this fresh water, 68.9% is in the form of ice and permanent snow cover in the Arctic, the Antarctic, and mountain glaciers. 30.8% is in the form of fresh groundwater. Only 0.3% of the fresh water on Earth is in easily accessible lakes, reservoirs and river systems. The total mass of the Earth's hydrosphere is about 1.4 × 1018 tonnes, which is about 0.023% of Earth's total mass. About 20 × 1012 tonnes of this is in Earth's atmosphere (for practical purposes, 1 cubic metre of water weighs one tonne). Approximately 75% of Earth's surface, an area of some 361 million square kilometers (139.5 million square miles), is covered by ocean. The average salinity of Earth's oceans is about 35 grams of salt per kilogram of sea water (3.5%). Fluid Liquid.
Water Cycle describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly constant over time but the partitioning of the water into the major reservoirs of ice, fresh water, saline water and atmospheric water is variable depending on a wide range of climatic variables. The water moves from one reservoir to another, such as from river to ocean, or from the ocean to the atmosphere, by the physical processes of evaporation, condensation, precipitation, infiltration, surface runoff, and subsurface flow. In doing so, the water goes through different forms: liquid, solid (ice) and vapor. The water cycle involves the exchange of energy, which leads to temperature changes. When water evaporates, it takes up energy from its surroundings and cools the environment. When it condenses, it releases energy and warms the environment. These heat exchanges influence climate. The evaporative phase of the cycle purifies water which then replenishes the land with freshwater. The flow of liquid water and ice transports minerals across the globe. It is also involved in reshaping the geological features of the Earth, through processes including erosion and sedimentation. The water cycle is also essential for the maintenance of most life and ecosystems on the planet. Hydrological Cycle of the earth is the sum total of all processes in which water moves from the land and ocean surface to the atmosphere and back in form of precipitation. The hydrological cycle is dependent on various factors and is equally affected by oceans and land surfaces. Water Cycle.
Condensation is the change of the physical state of matter from gas phase into liquid phase, and is the reverse of evaporation. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapour to liquid water when in contact with a liquid or solid surface or cloud condensation nuclei within the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition. Distill.
Dew Point is the temperature at which a given concentration of water vapor in air will form dew. More specifically it is a measure of atmospheric moisture. It is the temperature to which air must be cooled at constant pressure and water content to reach saturation. A higher dew point indicates more moisture in the air; a dew point greater than 20 °C (68 °F) is considered uncomfortable and greater than 22 °C (72 °F) is considered to be extremely humid. Frost point is the dew point when temperatures are below freezing. Weather (wind)
Humidity - Liquids - Viscosity - Heat - Boiling Water - Ice
Interesting Facts about Water - Memory - Interesting Information about Water.
Water Vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of Ice. Unlike other forms of water, water vapor is invisible. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is lighter than air and triggers convection currents that can lead to clouds.
Vaporization of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenomenon. Volatile.
Evaporation is a type of vaporization of a liquid that occurs from the surface of a liquid into a gaseous phase that is not saturated with the evaporating substance. The other type of vaporization is boiling, which is characterized by bubbles of saturated vapor forming in the liquid phase. Steam produced in a boiler is another example of evaporation occurring in a saturated vapor phase. Evaporation that occurs directly from the solid phase below the melting point, as commonly observed with ice at or below freezing or moth crystals (napthalene or paradichlorobenzene), is called sublimation, which is the phase transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition (deposition).
Melting - Transpiration - Heat (hot air)
Convection is the heat transfer due to bulk movement of molecules within fluids such as gases and liquids, including molten rock (rheid). Convection takes place through advection, diffusion or both. The movement caused within a fluid by the tendency of hotter and therefore less dense material to rise, and colder, denser material to sink under the influence of gravity, which consequently results in transfer of heat.
Advection is the transport of a substance by bulk motion. The properties of that substance are carried with it. Generally the majority of the advected substance is a fluid. The properties that are carried with the advected substance are conserved properties such as energy. An example of advection is the transport of pollutants or silt in a river by bulk water flow downstream. Another commonly advected quantity is energy or enthalpy. Here the fluid may be any material that contains thermal energy, such as water or air. In general, any substance or conserved, extensive quantity can be advected by a fluid that can hold or contain the quantity or substance. During advection, a fluid transports some conserved quantity or material via bulk motion. The fluid's motion is described mathematically as a vector field, and the transported material is described by a scalar field showing its distribution over space. Advection requires currents in the fluid, and so cannot happen in rigid solids. It does not include transport of substances by molecular diffusion. Advection is sometimes confused with the more encompassing process of convection which is the combination of advective transport and diffusive transport. In meteorology and physical oceanography, advection often refers to the transport of some property of the atmosphere or ocean, such as heat, humidity (see moisture) or salinity. Advection is important for the formation of orographic clouds and the precipitation of water from clouds, as part of the hydrological cycle.
Water Density achieves its maximum at 4 degrees Celsius or 39.2 degrees Fahrenheit. As water drops below that temperature, it becomes less dense, which is why ice floats. Density: 997 kg/m³.
Density of a substance is its mass per unit volume. Mass Concentration (wiki)
Relative Density is the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water at its densest (at 4 °C or 39.2 °F); for gases, air at room temperature (20 °C or 68 °F) is the reference. The term "relative density" is often preferred in scientific usage.
Dehydration - Electrolytes
Dehydration is a deficit of total body water, with an accompanying disruption of metabolic processes. Dehydration can also cause hypernatremia, which is a high sodium ion level in the blood. Dehydration is distinct from hypovolemia (loss of blood volume, particularly plasma). Dehydration occurs when free water loss exceeds free water intake, usually due to exercise or disease, but also due to high environmental temperature. Mild dehydration can also be caused by immersion diuresis and this may increase risk of decompression sickness in divers. Most people can tolerate a three to four percent decrease in total body water without difficulty or adverse health effects. A five to eight percent decrease can cause fatigue and dizziness. Loss of over ten percent of total body water can cause physical and mental deterioration, accompanied by severe thirst. Death occurs at a loss of between fifteen and twenty-five percent of the body water, or 12-18 pounds of water. Mild dehydration is characterized by thirst and general discomfort and is usually resolved with oral rehydration. A person can live about 3 days without water.
Electrolytes - Sweating (exercise or hot days)
Mouth is Dry. The darker the urine, the more dehydrated you are. Test the elasticity of your skin by pinching the back of your hand and hold it for a few seconds. Let go and if the little "tent" stays pinched and takes more than 5 seconds to go back to normal, it's usually a sign of moderate dehydration. Sugary drinks cause you to lose more body fluid. Sugary drinks create an acidic environment that can impair enzyme function and decrease your body’s water storage capacity, which is necessary to metabolize all the extra sugar. High-Protein intake causes the body has to use more water to metabolize the naturally occurring nitrogen in protein, and cells can become water-depleted. Salty Foods increase fluid loss in your body because water is needed to eliminate all the extra sodium naturally present in salt.
Xerostomia also known as dry mouth and dry mouth syndrome, is dryness in the mouth, which may be associated with a change in the composition of saliva, or reduced salivary flow, or have no identifiable cause. This symptom is very common and is often seen as a side effect of many types of medication. It is more common in older people (mostly because this group tend to take several medications) and in persons who breathe through their mouths (mouthbreathing). Dehydration, radiotherapy involving the salivary glands, chemotherapy and several diseases can cause hyposalivation or a change in saliva consistency and hence a complaint of xerostomia. Sometimes there is no identifiable cause, and there may be a psychogenic reason for the complaint.
Diuretic is any substance that promotes diuresis, that is, the increased production of urine. This includes forced diuresis. There are several categories of diuretics. All diuretics increase the excretion of water from bodies, although each class does so in a distinct way. Alternatively, an antidiuretic such as vasopressin, or antidiuretic hormone, is an agent or drug which reduces the excretion of water in urine.
Overhydrating (drinking too much water) Some people overly aggressively, out of a fear of dehydration, drink so much water that they dilute their blood and their brain swells. Dosage.
Antidiuretic Hormone is a chemical produced in the brain that causes the kidneys to release less water, decreasing the amount of urine produced. A high ADH level causes the body to produce less urine. A low level results in greater urine production.
Vasopressin is a hormone synthesized as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon of that cell, which terminates in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure. A third function is possible. Some AVP may be released directly into the brain from the hypothalamus, and may play an important role in social behavior, sexual motivation and pair bonding, and maternal responses to stress. Vasopressin induces differentiation of stem cells into cardiomyocytes and promotes heart muscle homeostasis.
Getting parched can fuzz attentiveness and make it harder to solve problems. Dehydration can easily put a dent in those and other cognitive functions.
Hyponatremia is a low sodium level in the blood. It is generally defined as a sodium concentration of less than 135 mmol/L (135 mEq/L), with severe hyponatremia being below 120 mEql/L. Symptoms can be absent, mild or severe. Mild symptoms include a decreased ability to think, headaches, nausea, and poor balance. Severe symptoms include confusion, seizures, and coma. The causes of hyponatremia are typically classified by a person's body fluid status into low volume, normal volume, or high volume. Low volume hyponatremia can occur from diarrhea, vomiting, diuretics, and sweating. Normal volume hyponatremia is divided into cases with dilute urine and concentrated urine. Cases in which the urine is dilute include adrenal insufficiency, hypothyroidism, and drinking too much water or too much beer. Cases in which the urine is concentrated include syndrome of inappropriate antidiuretic hormone secretion (SIADH). High volume hyponatremia can occur from heart failure, liver failure, and kidney failure. Conditions that can lead to falsely low sodium measurements include high blood protein levels such as in multiple myeloma, high blood fat levels, and high blood sugar. Treatment is based on the underlying cause. Correcting hyponatremia too quickly can lead to complications. Rapid partial correction with 3% normal saline is only recommended in those with significant symptoms and occasionally those in whom the condition was of rapid onset. Low volume hyponatremia is typically treated with intravenous normal saline. SIADH is typically treated with fluid restriction while high volume hyponatremia is typically treated with both fluid restriction and a diet low in salt. Correction should generally be gradual in those in whom the low levels have been present for more than two days. Hyponatremia occurs in about 20% of those admitted to hospital and 10% of people during or after an endurance sporting event. Among those in hospital, hyponatremia is associated with an increased risk of death. The economic costs of hyponatremia are estimated at $2.6 billion in the United States.
A Dad Didn't Brush His Teeth For 40 Days. This Is What Happened To His Kidneys (youtube)
Water Intoxication also known as water poisoning or hyperhydration, is a potentially fatal disturbance in brain functions that results when the normal balance of electrolytes in the body is pushed outside safe limits by overhydration.
Mapping the Neural Circuit Governing Thirst. Hierarchical Excitatory Neural Circuits That Drive Drinking. There are three regions in the mouse brain that are known to process thirst: the subfornical organ (SFO), the organum vasculosum laminae terminalis (OVLT), and the median preoptic nucleus (MnPO). Together, these regions form a sheet-like structure in the forebrain (near the front of the brain) called the lamina terminalis (LT). Most regions of the brain are protected by the nearly impenetrable blood-brain barrier, a layer of tightly packed cells that separates the bloodstream from the brain. But this is not the case for the SFO and OVLT -- they interface directly with a mouse's bloodstream, allowing the two regions to measure the sodium content, or saltiness, of the blood, which indicates the level of hydration. Therefore, the LT serves as the primary structure involved in thirst regulation. When you are dehydrated, you may gulp down water for several seconds and you feel satisfied. However, at that point your blood is not rehydrated yet: it usually takes about 10 to 15 minutes. Therefore, the SFO and the OVLT would not be able to detect blood rehydration soon after drinking. Nevertheless, the brain somehow knows when to stop drinking even before the body is fully rehydrated. Because of this temporal discrepancy between body rehydration and satiation signals in the brain, the researchers reasoned that some kind of rapid signal must be suppressing drinking behavior.
Transepidermal Water Loss is defined as the measurement of the quantity of water that passes from inside a body (animal or plant) through the epidermal layer (skin) to the surrounding atmosphere via diffusion and evaporation processes.
 Shenu: Hydrolemic System (video)
Shenu: Hydrolemic System (video)
SIPPO: Smart Cup Hydration made Easy
Electrolyte Imbalance. Electrolytes play a vital role in maintaining homeostasis within the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by the following mechanisms: excessive ingestion; diminished elimination of an electrolyte; diminished ingestion or excessive elimination of an electrolyte. The most serious electrolyte disturbances involve abnormalities in the levels of sodium, potassium or calcium. Other electrolyte imbalances are less common, and often occur in conjunction with major electrolyte changes. Chronic laxative abuse or severe diarrhea or vomiting (gastroenteritis) can lead to electrolyte disturbances along with dehydration. People suffering from bulimia or anorexia nervosa are at especially high risk for an electrolyte imbalance.
Nuun Performance Hydration (amazon) Sodium: 380 mg - Potassium: 200-210 mg - Magnesium: 20 mg - Calcium: 15 mg. Chloride: 80 mg - 15 grams of carbohydrates. Ingredients: Dextrose, Cane Sugar (vegan), Dried Fruit Powder, Sodium Citrate, Citric Acid, Potassium Citrate, Potassium Chloride, Magnesium Citrate, Calcium Citrate.
Electrolytes in Batteries.
Fluid Balance is an aspect of the homeostasis of living organisms in which the amount of water in the organism needs to be controlled, via osmoregulation and behavior, such that the concentrations of electrolytes (salts in solution) in the various body fluids are kept within healthy ranges. The core principle of fluid balance is that the amount of water lost from the body must equal the amount of water taken in; for example, in human homeostasis, the output (via respiration, perspiration, urination, defecation, and expectoration) must equal the input (via eating, drinking, and parenteral intake). Euvolemia is the state of normal body fluid volume, including blood volume, interstitial fluid volume, and intracellular fluid volume; hypovolemia and hypervolemia are imbalances. Water is necessary for all life on Earth. Humans can survive for 4 to 6 weeks without food but only for a few days without water. Profuse Sweating can increase the need for electrolyte replacement. Water-electrolyte imbalance produces headache and fatigue if mild; illness if moderate, and sometimes even death if severe. For example, water intoxication (which results in hyponatremia), the process of consuming too much water too quickly, can be fatal. Deficits to body water result in volume contraction and dehydration. Diarrhea is a threat to both body water volume and electrolyte levels, which is why diseases that cause diarrhea are great threats to fluid balance.
Thirst is the craving for fluids, resulting in the basic instinct of animals to drink. It is an essential mechanism involved in fluid balance. It arises from a lack of fluids or an increase in the concentration of certain osmolites, such as salt. If the water volume of the body falls below a certain threshold or the osmolite concentration becomes too high, the brain signals thirst. Continuous dehydration can cause many problems, but is most often associated with renal problems and neurological problems such as seizures. Excessive thirst, known as polydipsia, along with excessive urination, known as polyuria, may be an indication of diabetes mellitus or diabetes insipidus. There are receptors and other systems in the body that detect a decreased volume or an increased osmolite concentration. They signal to the central nervous system, where central processing succeeds. Some sources, therefore, distinguish "extracellular thirst" from "intracellular thirst", where extracellular thirst is thirst generated by decreased volume and intracellular thirst is thirst generated by increased osmolite concentration. Nevertheless, the craving itself is something generated from central processing in the brain, no matter how it is detected.
Thirst Regulation is related to two types of brain cells, one that responds to a decline in fluid in our bodies, while the other monitors levels of salt and other minerals. These specialized thirst cells seem to determine whether animals and people crave pure water or some other drink that contains salt and other minerals.
The cellular basis of distinct thirst modalities. Fluid intake is an essential innate behaviour that is mainly caused by two distinct types of thirst1–3. Increased blood osmolality induces osmotic thirst that drives animals to consume pure water. Conversely, the loss of body fluid induces hypovolaemic thirst, in which animals seek both water and minerals (salts) to recover blood volume. Circumventricular organs in the lamina terminalis are critical sites for sensing both types of thirst-inducing stimulus4–6. However, how different thirst modalities are encoded in the brain remains unknown. Here we employed stimulus-to-cell-type mapping using single-cell RNA sequencing to identify the cellular substrates that underlie distinct types of thirst. These studies revealed diverse types of?excitatory and inhibitory neuron in each circumventricular organ structure. We show that unique combinations of these?neuron types are activated under osmotic and hypovolaemic stresses.
Integration of Hypernatremia and Angiotensin II by the Organum Vasculosum of the Lamina Terminalis Regulates Thirst. The organum vasculosum of the lamina terminalis (OVLT) contains NaCl-sensitive neurons to regulate thirst, neuroendocrine function, and autonomic outflow. The OVLT also expresses the angiotensin II (AngII) type1 receptor, and AngII increases Fos expression in OVLT neurons. The present study tested whether individual OVLT neurons sensed both NaCl and AngII to regulate thirst and body fluid homeostasis.
Urine - Water Therapy
A full bladder is about the size of a soft ball: When your bladder is full, holding up to 800 cubic centimeters of fluid, or 27.0512 Fluid Ounces.
Drinking Room Temperature Water - Drinking Cold Water
Drink cold water to cool down. Drink room temperature water for digestion, detox, and pain relief. When you drink cold water, your blood vessels shrink, and this restricts your digestion. Warm water helps break down food, aids constipation, and even helps you lose weight while improving your blood circulation. Room temperature can even help stop pains like a headache. Cold water is not bad for you or better for you, is just that the different water temperatures help your body in different ways. When cold water hits the stomach, the body is forced to use energy in order to warm up that liquid inside your body to match that of the body's natural temperature. Instead of working to extract all the foods nutrients, your digestive system is instead working on regulating the temperature of the cold drink. Water between 50 and 72 degrees allows our bodies to rehydrate faster because it is absorbed more quickly. Drinking warm water increases blood circulation along with protecting internal organs from damage. This is why most health professionals recommend drinking warm water for optimal health. However, on a hot day, you can drink cold water as it cools your body temperature quickly. Body Temperatures - Ice Water Therapy.
Body Water is the water content of an animal body that is contained in the tissues, the blood, the bones and elsewhere. This water makes up a significant fraction of the human body, both by weight and by volume. Ensuring the right amount of body water is part of fluid balance, an aspect of homeostasis. The average human adult male is approximately 69% water, by weight.
The average human being consists of about 7 x 1027 atoms (7,000 trillion trillion atoms) — 65 percent oxygen, 18 percent carbon, 10 percent hydrogen, 3 percent nitrogen, 1.4 percent calcium, 1.1 percent phosphorous, and traces of 54 other chemical elements.
Composition of the Human Body (wiki)
Hydrocephalus is an abnormal accumulation of cerebrospinal fluid (CSF) in the brain.
Cerebrospinal Fluid is a clear, colorless body fluid found in the brain and spine. It is produced in the choroid plexuses of the ventricles of the brain. It acts as a cushion or buffer for the brain's cortex, providing basic mechanical and immunological protection to the brain inside the skull. The CSF also serves a vital function in cerebral autoregulation of cerebral blood flow.
Body Fluid bodily fluids or biofluids are liquids originating from inside the bodies of living people. They include fluids that are excreted or secreted from the body as well as body water that normally is not. (Drool) The dominating content of body fluids is body water. Approximately 60-65% of body water is contained within the cells (in intracellular fluid) with the other 35-40% of body water contained outside the cells (in extracellular fluid). This fluid component outside the cells includes the fluid between the cells (interstitial fluid), lymph and blood. There are approximately 6 to 10 liters of lymph in the body, compared to 3.5 to 5 liters of blood.
Liquids - Fluids
Liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. Liquid is one of the four fundamental states of matter, with the others being solid, gas, and plasma, and is the only state with a definite volume but no fixed shape. A liquid is made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds. Water is by far the most common liquid on Earth. Like a gas, a liquid is able to flow and take the shape of a container. Most liquids resist compression, although others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension, leading to wetting phenomena. The density of a liquid is usually close to that of a solid, and much higher than in a gas. Therefore, liquid and solid are both termed condensed matter. On the other hand, as liquids and gases share the ability to flow, they are both called fluids. Although liquid water is abundant on Earth, this state of matter is actually the least common in the known universe, because liquids require a relatively narrow temperature/pressure range to exist. Most known matter in the universe is in gaseous form (with traces of detectable solid matter) as interstellar clouds or in plasma form within stars. Phases of Water.
Hydrophobe is the physical property of a molecule, known as a hydrophobe, that is seemingly repelled from a mass of water. (Strictly speaking, there is no repulsive force involved; it is an absence of attraction.) In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. Hydrophobic is often used interchangeably with lipophilic, "fat-loving". However, the two terms are not synonymous. While hydrophobic substances are usually lipophilic, there are exceptions, such as the silicones and fluorocarbons. Oil floats to the top of water because it is less dense than water. Oil and water don't mix because water molecules are more attracted to each other than to oil molecules.
Water Basics - Surface Tension - Viscosity - Water in a Vacuum - Sound Shapes
Hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water. In contrast, hydrophobes are not attracted to water and may seem to be repelled by it. Hydrophilic is having a tendency to mix with, dissolve in, or be wetted by water. Hydrolysis (electrolysis).
Amphiphile is a chemical compound possessing both hydrophilic (water-loving, polar) and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. This forms the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism. Organic compounds containing hydrophilic groups at both ends of a prolate (in the aggregate) molecule are called bolaamphiphilic. Common amphiphilic substances are soaps, detergents and lipoproteins.
Hydrophobic Effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the hydrophobic effect is essential to life. Substances for which this effect is observed are known as hydrophobes. The hydrophilic groups prevent phase separation of the molecules by maintaining the hydrophobic groups in water through formation of strong hydrogen bonds with water molecules. The driving force for this self-assembly is the hydrophobic effect. Wax.
Micro-Electro-Fluidic Probe (MeFP) to isolate and pattern cells. Researchers have developed a dielectrophoresis (DEP) enabled MicroelectroFluidic Probe (MeFP) that has the ability to sequentially separate and pattern mammalian cells in an open microfluidic system.
Dielectrophoresis is a phenomenon in which a force is exerted on a dielectric particle when it is subjected to a non-uniform electric field. This force does not require the particle to be charged. All particles exhibit dielectrophoretic activity in the presence of electric fields. However, the strength of the force depends strongly on the medium and particles electrical properties, on the particles shape and size, as well as on the frequency of the electric field. Consequently, fields of a particular frequency can manipulate particles with great selectivity. This has allowed, for example, the separation of cells or the orientation and manipulation of nanoparticles and nanowires. Furthermore, a study of the change in DEP force as a function of frequency can allow the electrical (or electrophysiological in the case of cells) properties of the particle to be elucidated.
New aspect to the way that DNA binds itself, and the role played by hydrophobic effects. DNA is constructed of two strands, consisting of sugar molecules and phosphate groups. Between these two strands are nitrogen bases, the compounds which make up organisms’ genes, with hydrogen bonds between them. Those hydrogen bonds have sometimes been seen as crucial to holding the two strands together.
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another (cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). The forces that cause adhesion and cohesion can be divided into several types. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are also certain emergent mechanical effects.
Cohesion in chemistry is the action or property of like molecules sticking together, being mutually attractive. It is an intrinsic property of a substance that is caused by the shape and structure of its molecules, which makes the distribution of orbiting electrons irregular when molecules get close to one another, creating electrical attraction that can maintain a microscopic structure such as a water drop. In other words, cohesion allows for surface tension, creating a "solid-like" state upon which light-weight or low-density materials can be placed. Capillary.
Coalesce is to come together to form one mass or whole. Combine (elements) in a mass or whole.
Emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable). Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase).
Dispersion in chemistry is a system in which discrete particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter. They are different from solutions, where dissolved molecules do not form a separate phase from the solute. Dispersions are classified in a number of different ways, including how large the particles are in relation to the particles of the continuous phase, whether or not precipitation occurs, and the presence of Brownian motion. In general, dispersions of particles sufficiently large for sedimentation are called suspensions, while those of smaller particles are called colloids.
Homogeneous and Heterogeneous Mixtures. A homogeneous mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components throughout any given sample. Homogeneity and Heterogeneity are concepts often used in the sciences and statistics relating to the uniformity in a substance or organism.
Laminar Flow is characterized by fluid particles following smooth paths in layers, with each layer moving smoothly past the adjacent layers with little or no mixing. At low velocities, the fluid tends to flow without lateral mixing, and adjacent layers slide past one another like playing cards. In fluid dynamics laminar flow occurs when a fluid flows in parallel layers, with no disruption between the layers. There are no cross-currents perpendicular to the direction of flow, nor eddies or swirls of fluids. In laminar flow, the motion of the particles of the fluid is very orderly with particles close to a solid surface moving in straight lines parallel to that surface. Laminar flow is a flow regime characterized by high momentum diffusion and low momentum convection. When a fluid is flowing through a closed channel such as a pipe or between two flat plates, either of two types of flow may occur depending on the velocity and viscosity of the fluid: laminar flow or turbulent flow. Laminar flow tends to occur at lower velocities, below a threshold at which it becomes turbulent. Turbulent flow is a less orderly flow regime that is characterized by eddies or small packets of fluid particles, which result in lateral mixing. In non-scientific terms, laminar flow is smooth, while turbulent flow is rough. Making a Laminar Flow Nozzle (youtube) - Fire Hose (wiki).
Bubble Ring is an underwater vortex ring where an air bubble occupies the core of the vortex, forming a ring shape. The ring of air as well as the nearby water spins poloidally as it travels through the water, much like a flexible bracelet might spin when it is rolled on to a person's arm. The faster the bubble ring spins, the more stable it becomes. Bubble rings and smoke rings are both examples of vortex rings—the physics of which is still under active study in fluid dynamics. Devices have been invented which generate bubble vortex rings.
Venturi Effect is the reduction in fluid pressure that results when a fluid flows through a constricted section (or choke) of a pipe. The Venturi effect is named after its discoverer, Giovanni Battista Venturi. In fluid dynamics, an incompressible fluid's velocity must increase as it passes through a constriction in accord with the principle of mass continuity, while its static pressure must decrease in accord with the principle of conservation of mechanical energy (Bernoulli's principle). Thus, any gain in kinetic energy a fluid may attain by its increased velocity through a constriction is balanced by a drop in pressure.
Light Non-Aqueous Phase Liquid is a groundwater contaminant that is not soluble in water and has lower density than water, in contrast to a DNAPL which has higher density than water. Once a LNAPL infiltrates the ground, it will stop at the height of the water table because the LNAPL is less dense than water. A light non-aqueous phase liquid (LNAPL) is a groundwater contaminant that is not soluble in water and has lower density than water, in contrast to a DNAPL which has higher density than water. Once a LNAPL infiltrates the ground, it will stop at the height of the water table because the LNAPL is less dense than water. Efforts to locate and remove LNAPLs is relatively less expensive and easier than for DNAPLs because LNAPLs float on top of the water in the underground water table. Examples of LNAPLs are benzene, toluene, xylene, and other hydrocarbons.
Dense Non-Aqueous Phase Liquid is a denser-than-water NAPL, i.e. a liquid that is both denser than water and is immiscible in or does not dissolve in water. The term DNAPL is used primarily by environmental engineers and hydrogeologists to describe contaminants in groundwater, surface water and sediments. DNAPLs tends to sink below the water table when spilled in significant quantities and only stop when they reach impermeable bedrock. Their penetration into an aquifer makes them difficult to locate and remediate. Examples of materials that are DNAPLs when spilled include: chlorinated solvents, such as trichloroethylene, tetrachloroethene, 1,1,1-trichloroethane and carbon tetrachloride. coal tar, creosote, polychlorinated biphenyl (PCBs), mercury, extra heavy crude oil, with an API gravity of less than 10.
Hygroscopy is the phenomenon of attracting and holding water molecules from the surrounding environment, which is usually at normal or room temperature. This is achieved through either absorption or adsorption with the adsorbing substance becoming physically changed somewhat. This could be an increase in volume, boiling point, viscosity, or other physical characteristic or property of the substance, as water molecules can become suspended between the substance's molecules in the process.
Desiccant is a hygroscopic substance that induces or sustains a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams. Porous - Refrigeration.
Humectant is a hygroscopic substance used to keep things moist; it is the opposite of a desiccant because it is wet. It is often a molecule with several hydrophilic groups, most often hydroxyl groups; however, amines and carboxyl groups, sometimes esterified, can be encountered as well (its affinity to form hydrogen bonds with molecules of water is the crucial trait). They are used in many products, including food, cosmetics, medicines and pesticides. A humectant attracts and retains the moisture in the air nearby via absorption, drawing the water vapor into or beneath the organism's or object's surface. When used as a food additive, a humectant has the effect of keeping the foodstuff moist. Humectants are sometimes used as a component of antistatic coatings for plastics. In pharmaceuticals and cosmetics, humectants can be used in topical dosage forms to increase the solubility of a chemical compound's active ingredients, increasing the active ingredients' ability to penetrate skin, or its activity time. This hydrating property can also be needed to counteract a dehydrating active ingredient (e.g., soaps, corticoids, and some alcohols), which is why humectants are common ingredients in a wide range of cosmetic and personal care products that make moisturization claims (e.g., hair conditioners, body lotions, face or body cleansers, lip balms, and eye creams).
Schlieren Photography is a visual process that is used to photograph the flow of fluids of varying density. Light Bending.
Magnetic Ionic Liquid can be obtained from 1-butyl-3-methylimidazolium chloride and ferric chloride. It has quite low water solubility an thus can be considered as hydrophobic ionic liquid.
Ionic Liquid is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions and short-lived ion pairs. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. They are known as "solvents of the future" as well as "designer solvents". Siphon.
Surface Tension
Surface Tension is the elastic tendency of a fluid surface which makes it acquire the least surface area possible. Surface tension allows insects (e.g. water striders), usually denser than water, to float and stride on a water surface. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). The net effect is an inward force at its surface that causes the liquid to behave as if its surface were covered with a stretched elastic membrane. Thus, the surface becomes under tension from the imbalanced forces, which is probably where the term "surface tension" came from. Because of the relatively high attraction of water molecules for each other through a web of hydrogen bonds, water has a higher surface tension (72.8 millinewtons per meter at 20 °C) compared to that of most other liquids. Surface tension is an important factor in the phenomenon of capillarity. Surface tension has the dimension of force per unit length, or of energy per unit area. The two are equivalent, but when referring to energy per unit of area, it is common to use the term surface energy, which is a more general term in the sense that it applies also to solids. In materials science, surface tension is used for either surface stress or surface free energy. Surface Tension tighter molecule bonds at the surface (Khana) - Viscosity.
Surface Energy quantifies the disruption of intermolecular bonds that occurs when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material (the molecules on the surface have more energy compared with the molecules in the bulk of the material), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material (see sublimation). The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk, or it is the work required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cut a bulk sample, creating two surfaces. Cutting a solid body into pieces disrupts its bonds, and therefore increases free energy. If the cutting is done reversibly, then conservation of energy means that the energy consumed by the cutting process will be equal to the energy inherent in the two new surfaces created. The unit surface energy of a material would therefore be half of its energy of cohesion, all other things being equal; in practice, this is true only for a surface freshly prepared in vacuum. Surfaces often change their form away from the simple "cleaved bond" model just implied above. They are found to be highly dynamic regions, which readily rearrange or react, so that energy is often reduced by such processes as passivation or adsorption.
Buoyancy or upthrust, is an upward force exerted by a fluid that opposes the weight of an immersed object. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus the pressure at the bottom of a column of fluid is greater than at the top of the column. Similarly, the pressure at the bottom of an object submerged in a fluid is greater than at the top of the object. The pressure difference results in a net upward force on the object. The magnitude of the force is proportional to the pressure difference, and (as explained by Archimedes' principle) is equivalent to the weight of the fluid that would otherwise occupy the volume of the object, i.e. the displaced fluid. For this reason, an object whose average density is greater than that of the fluid in which it is submerged tends to sink. If the object is less dense than the liquid, the force can keep the object afloat. This can occur only in a non-inertial reference frame, which either has a gravitational field or is accelerating due to a force other than gravity defining a "downward" direction. The center of buoyancy of an object is the centroid of the displaced volume of fluid.
Water Droplets Bounce on Water Surface. At the water's surface, because the molecules are restricted from moving upward anymore, hydrogen bonds are slightly more prevalent. This is what causes water to bead up, and is also resulting in the bouncing effect here. The drops have enough of their own surface tension to bounce off of the water below. Each time a drop does merge, about half the water gets tossed back up in a smaller droplet. This phenomenon is called the coalescence cascade.
Coalescence is the process by which two or more droplets, bubbles or particles merge during contact to form a single daughter droplet, bubble or particle. It can take place in many processes, ranging from meteorology to astrophysics. For example, it is seen in the formation of raindrops as well as planetary and star formation. In meteorology, its role is crucial in the formation of Rain. As droplets are carried by the updrafts and downdrafts in a cloud, they collide and coalesce to form larger droplets. When the droplets become too large to be sustained on the air currents, they begin to fall as rain. Adding to this process, the cloud may be seeded with ice from higher altitudes, either via the cloud tops reaching −40 °C (−40 °F), or via the cloud being seeded by ice from cirrus clouds. In cloud physics the main mechanism of collision is the different terminal velocity between the droplets. The terminal velocity is a function of the droplet size. The other factors that determine the collision rate are the droplet concentration and turbulence. Rain.
Elastic Collision is an encounter between two bodies in which the total kinetic energy of the two bodies remains the same. In an ideal, perfectly elastic collision, there is no net conversion of kinetic energy into other forms such as heat, noise, or potential energy. During the collision of small objects, kinetic energy is first converted to potential energy associated with a repulsive force between the particles (when the particles move against this force, i.e. the angle between the force and the relative velocity is obtuse), then this potential energy is converted back to kinetic energy (when the particles move with this force, i.e. the angle between the force and the relative velocity is acute).
Marangoni Effect is the mass transfer along an interface between two fluids due to a gradient of the surface tension. In the case of temperature dependence, this phenomenon may be called thermo-capillary convection (or Bénard–Marangoni convection).
Tears of Wine is manifested as a ring of clear liquid, near the top of a glass of wine, from which droplets continuously form and drop back into the wine. It is most readily observed in a wine which has a high alcohol content. It is also referred to as wine legs, "fingers", curtains, or church windows.
Archimedes' Principle states that the upward buoyant force that is exerted on a body immersed in a fluid, whether fully or partially submerged, is equal to the weight of the fluid that the body displaces and acts in the upward direction at the center of mass of the displaced fluid. Archimedes' principle is a law of physics fundamental to fluid mechanics. It was formulated by Archimedes of Syracuse.
Water Thread Experiment is a phenomenon that occurs when two containers of deionized water, placed on an insulator, are connected by a thread, then a high-voltage positive electric charge is applied to one container, and a negative charge to the other. At a critical voltage, an unsupported water liquid bridge is formed between the containers, which will remain even when they are separated. The phenomenon was first reported in 1893 in a public lecture by the British engineer William Armstrong.
Sand Castles stay together because the water forms capillary bridges between the sand grains that hold them together. These capillary bridges can be quite strong, allowing construction of complex castles.
Friction (drag)
Capillary Bridges are created between two rigid bodies with an arbitrary shape with a minimized surface of liquid or membrane. Capillary bridges also may form between two liquids. Plateau defined a sequence of capillary shapes known as (1) nodoid with 'neck', (2) catenoid, (3) unduloid with 'neck', (4) cylinder, (5) unduloid with 'haunch' (6) sphere and (7) nodoid with 'haunch'. The presence of capillary bridge, depending on their shapes, can lead to attraction or repulsion between the solid bodies. The simplest cases of them are the axisymmetric ones. We distinguished three important classes of bridging, depending on connected bodies surface shapes: Capillary bridges and their properties may also be influenced by Earth gravity and by properties of the bridged surfaces. The bridging substance may be a liquid or a gas. The enclosing boundary is called the interface (capillary surface). The interface is characterized by a particular surface tension.
Fluid Mechanics
 Fluid Mechanics
is the branch of physics that studies the mechanics of fluids (liquids,
gases, and plasmas) and the forces on them. Fluid mechanics has a wide
range of applications, including for mechanical engineering, chemical
engineering, geophysics, astrophysics, and biology. Fluid mechanics can be
divided into fluid statics, the study of fluids at rest; and fluid
dynamics, the study of the effect of forces on fluid motion.
Fluid Dynamics
is a subdiscipline of fluid mechanics that describes the flow of fluids -
liquids and gases. Hydraulics (engineering).
Chocolate Lullaby -
Music Video (youtube).
Fluid Mechanics
is the branch of physics that studies the mechanics of fluids (liquids,
gases, and plasmas) and the forces on them. Fluid mechanics has a wide
range of applications, including for mechanical engineering, chemical
engineering, geophysics, astrophysics, and biology. Fluid mechanics can be
divided into fluid statics, the study of fluids at rest; and fluid
dynamics, the study of the effect of forces on fluid motion.
Fluid Dynamics
is a subdiscipline of fluid mechanics that describes the flow of fluids -
liquids and gases. Hydraulics (engineering).
Chocolate Lullaby -
Music Video (youtube).Fluid Dynamics is a subdiscipline of fluid mechanics that deals with fluid flow.
Volumetric image of a helical vortex leapfrogging through a vortex ring in water, with dye-blob tracks overlaid in warm colors.
Eddy is a circular movement of water, counter to a main current, causing a small whirlpool. Eddy in fluid dynamics is the swirling of a fluid and the reverse current created when the fluid is in a turbulent flow regime. The moving fluid creates a space devoid of downstream-flowing fluid on the downstream side of the object. Fluid behind the obstacle flows into the void creating a swirl of fluid on each edge of the obstacle, followed by a short reverse flow of fluid behind the obstacle flowing upstream, toward the back of the obstacle. This phenomenon is naturally observed behind large emergent rocks in swift-flowing rivers. Vortex.
Hydraulic Jump is a phenomenon in the science of hydraulics which is frequently observed in open channel flow such as rivers and spillways. When liquid at high velocity discharges into a zone of lower velocity, a rather abrupt rise occurs in the liquid surface. The rapidly flowing liquid is abruptly slowed and increases in height, converting some of the flow's initial kinetic energy into an increase in potential energy, with some energy irreversibly lost through turbulence to heat. In an open channel flow, this manifests as the fast flow rapidly slowing and piling up on top of itself similar to how a shockwave forms. What is a Hydraulic Jump? (youtube) - Hydraulic Jumps in Rectangular Channels is a natural phenomenon that occurs whenever flow changes from supercritical to subcritical flow. In this transition, the water surface rises abruptly, surface rollers are formed, intense mixing occurs, air is entrained, and often a large amount of energy is dissipated. Dams (hydro energy).
Hydraulic Ram is a cyclic water pump powered by hydropower. It takes in water at one "hydraulic head" (pressure) and flow rate, and outputs water at a higher hydraulic head and lower flow rate. The device uses the water hammer effect to develop pressure that allows a portion of the input water that powers the pump to be lifted to a point higher than where the water originally started. The hydraulic ram is sometimes used in remote areas, where there is both a source of low-head hydropower and a need for pumping water to a destination higher in elevation than the source. In this situation, the ram is often useful, since it requires no outside source of power other than the kinetic energy of flowing water. Head Pressure - Elevation Pressure - Velocity Head. Siphoning.
Check Valve is a valve that normally allows fluid (liquid or gas) to flow through it in only one direction.
Water Hammer is a pressure surge or wave caused when a fluid (usually a liquid but sometimes also a gas) in motion is forced to stop or change direction suddenly (momentum change). A water hammer commonly occurs when a valve closes suddenly at an end of a pipeline system, and a pressure wave propagates in the pipe. It is also called hydraulic shock. This pressure wave can cause major problems, from noise and vibration to pipe collapse. It is possible to reduce the effects of the water hammer pulses with accumulators, expansion tanks, surge tanks, blowoff valves, and other features. Rough calculations can be made either using the Zhukovsky (Joukowsky) equation, or more accurate ones using the method of characteristics. What is Water Hammer? (youtube).
Air Lock is a restriction of, or complete stoppage of liquid flow caused by vapour trapped in a high point of a liquid-filled pipe system. The gas, being less dense than the liquid, rises to any high points. This phenomenon is known as vapor lock, or air lock. Flushing the system with high flow or pressures can help move the gas away from the highest point, or a tap (or automatic vent valve) can be installed to let the gas out.
Air Gap in plumbing is the unobstructed vertical space between the water outlet and the flood level of a fixture. Air gaps of appropriate design are required for water safety by legislation in many countries. A simple example is the space between a wall mounted faucet and the sink rim (this space is the air gap). Water can easily flow from the faucet into the sink, but there is no way that water can flow from the sink into the faucet without modifying the system. This arrangement will prevent any contaminants in the sink from flowing into the potable water system by siphonage and is the least expensive form of backflow prevention.
Volumetric Flow Rate is the volume of fluid which passes per unit time. Air Flow.
Discharge in hydrology is the volumetric flow rate of water that is transported through a given cross-sectional area.
Flow Measurement is the quantification of bulk fluid movement. Drip.
Laminar Flow
Plateau–Rayleigh Instability explains why and how a falling stream of fluid breaks up into smaller packets with the same volume but less surface area. It is related to the Rayleigh–Taylor instability and is part of a greater branch of fluid dynamics concerned with fluid thread breakup. This fluid instability is exploited in the design of a particular type of ink jet technology whereby a jet of liquid is perturbed into a steady stream of droplets. The driving force of the Plateau–Rayleigh instability is that liquids, by virtue of their surface tensions, tend to minimize their surface area. A considerable amount of work has been done recently on the final pinching profile by attacking it with self-similar solutions.
Saddle Point is a point on the surface of the graph of a function where the slopes (derivatives) in orthogonal directions are all zero (a critical point), but which is not a local extremum of the function.
Hydrostatics is the branch of fluid mechanics that studies incompressible fluids at rest. The branch of science concerned with forces acting on or exerted by fluids or liquids. It encompasses the study of the conditions under which fluids are at rest in stable equilibrium as opposed to fluid dynamics, the study of fluids in motion. Hydrostatics are categorized as a part of the fluid statics, which is the study of all fluids, incompressible or not, at rest. Hydrostatics is fundamental to hydraulics, the engineering of equipment for storing, transporting and using fluids. It is also relevant to geophysics and astrophysics (for example, in understanding plate tectonics and the anomalies of the Earth's gravitational field), to meteorology, to medicine (in the context of blood pressure), and many other fields. Hydrostatics offers physical explanations for many phenomena of everyday life, such as why atmospheric pressure changes with altitude, why wood and oil float on water, and why the surface of still water is always level.
Hydrodynamics is a branch of physics that deals with the motion of fluids and the forces acting on solid bodies immersed in fluids and in motion relative to them.
Entrainment in hydrodynamics is the transport of fluid across an interface between two bodies of fluid by a shear induced turbulent flux.
Compressible Flow is the branch of fluid mechanics that deals with flows having significant changes in fluid density. While all flows are compressible, flows are usually treated as being incompressible when the Mach number (the ratio of the speed of the flow to the speed of sound) is less than 0.3 (since the density change due to velocity is about 5% in that case). The study of compressible flow is relevant to high-speed aircraft, jet engines, rocket motors, high-speed entry into a planetary atmosphere, gas pipelines, commercial applications such as abrasive blasting, and many other fields.
Capillary Action
Capillary Action sometimes capillarity, capillary motion, or wicking, is the ability of a liquid to flow in narrow spaces without the assistance of, or even in opposition to, external forces like gravity. The effect can be seen in the drawing up of liquids between the hairs of a paint-brush, in a thin tube, in porous materials such as paper and plaster, in some non-porous materials such as sand and liquefied carbon fiber, or in a cell. It occurs because of intermolecular forces between the liquid and surrounding solid surfaces. If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and adhesive forces between the liquid and container wall act to lift the liquid. Ceramic Water Spikes.
Capillary Condensation is the process by which multilayer adsorption from the vapor [phase] into a porous medium proceeds to the point at which pore spaces become filled with condensed liquid from the vapor [phase]. The unique aspect of capillary condensation is that vapor condensation occurs below the saturation vapor pressure, Psat, of the pure liquid. This result is due to an increased number of van der Waals interactions between vapor phase molecules inside the confined space of a capillary. Once condensation has occurred, a meniscus immediately forms at the liquid-vapor interface which allows for equilibrium below the saturation vapor pressure. Meniscus formation is dependent on the surface tension of the liquid and the shape of the capillary, as shown by the Young-Laplace equation. As with any liquid-vapor interface involving a meniscus, the Kelvin equation provides a relation for the difference between the equilibrium vapor pressure and the saturation vapor pressure. A capillary does not necessarily have to be a tubular, closed shape, but can be any confined space with respect to its surroundings. Capillary condensation is an important factor in both naturally occurring and synthetic porous structures. In these structures, scientists use the concept of capillary condensation to determine pore size distribution and surface area through adsorption isotherms.Synthetic applications such as sintering of materials are also highly dependent on bridging effects resulting from capillary condensation. In contrast to the advantages of capillary condensation, it can also cause many problems in materials science applications such as atomic-force microscopy and microelectromechanical systems.
Porous is something that is full of pores or vessels or holes that is able to absorb fluids and allow passage in and out.
Porous Medium is a material containing pores (voids). The skeletal portion of the material is often called the "matrix" or "frame". Insulation - Bones.
Porosity is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Filtering - Membranes - Metal Ions - Metal-Organic Framework.
Permeability is a measure of the ability of a porous material (often, a rock or an unconsolidated material) to allow fluids to pass through it. The permeability of a medium is related to the porosity, but also to the shapes of the pores in the medium and their level of connectedness.
Semipermeable Membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by diffusion—or occasionally by more specialized processes of facilitated diffusion, passive transport or active transport. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials thicker than a membrane are also semipermeable. One example of this is the thin film on the inside of the egg. Note that a semipermeable membrane is not the same as a selectively permeable membrane. Semipermeable membrane describes a membrane that allows some particles to pass through (by size), whereas the selectively permeable membrane "chooses" what passes through (size is not a factor).
Molecular Sieve is a material with pores (very small holes) of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrate through the stationary bed of porous, semi-solid substance referred to as a sieve (or matrix), the components of highest molecular weight (which are unable to pass into the molecular pores) leave the bed first, followed by successively smaller molecules. Some molecular sieves are used in chromatography, a separation technique that sorts molecules based on their size. Other molecular sieves are used as desiccants (some examples include activated charcoal and silica gel). The diameter of a molecular sieve is measured in ångströms (Å) or nanometres (nm). According to IUPAC notation, microporous materials have pore diameters of less than 2 nm (20 Å) and macroporous materials have pore diameters of greater than 50 nm (500 Å); the mesoporous category thus lies in the middle with pore diameters between 2 and 50 nm (20–500 Å). Liquid Fluid.
Flux describes the quantity which passes through a surface or substance.
Osmosis is the spontaneous net movement of solvent molecules through a semi-permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a semipermeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work.
Diffusion is the net movement of molecules or atoms from a region of high concentration with high chemical potential to a region of low concentration with low chemical potential. This is also referred to as the movement of a substance down a concentration gradient. A gradient is the change in the value of a quantity e.g. concentration, pressure, or temperature with the change in another variable, usually distance. A change in concentration over a distance is called a concentration gradient, a change in pressure over a distance is called a pressure gradient, and a change in temperature over a distance is a called a temperature gradient.
Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. Water is necessary for plants but only a small amount of water taken up by the roots is used for growth and metabolism. The remaining 97–99.5% is lost by transpiration and guttation. Leaf surfaces are dotted with pores called stomata, and in most plants they are more numerous on the undersides of the foliage. The stomata are bordered by guard cells and their stomatal accessory cells (together known as stomatal complex) that open and close the pore. Transpiration occurs through the stomatal apertures, and can be thought of as a necessary "cost" associated with the opening of the stomata to allow the diffusion of carbon dioxide gas from the air for photosynthesis. Transpiration also cools plants, changes osmotic pressure of cells, and enables mass flow of mineral nutrients and water from roots to shoots. Two major factors influence the rate of water flow from the soil to the roots: the hydraulic conductivity of the soil and the magnitude of the pressure gradient through the soil. Both of these factors influence the rate of bulk flow of water moving from the roots to the stomatal pores in the leaves via the xylem. Mass flow of liquid water from the roots to the leaves is driven in part by capillary action, but primarily driven by water potential differences. If the water potential in the ambient air is lower than the water potential in the leaf airspace of the stomatal pore, water vapor will travel down the gradient and move from the leaf airspace to the atmosphere. This movement lowers the water potential in the leaf airspace and causes evaporation of liquid water from the mesophyll cell walls. This evaporation increases the tension on the water menisci in the cell walls and decrease their radius and thus the tension that is exerted on the water in the cells. Because of the cohesive properties of water, the tension travels through the leaf cells to the leaf and stem xylem where a momentary negative pressure is created as water is pulled up the xylem from the roots. In taller plants and trees, the force of gravity can only be overcome by the decrease in hydrostatic (water) pressure in the upper parts of the plants due to the diffusion of water out of stomata into the atmosphere. Water is absorbed at the roots by osmosis, and any dissolved mineral nutrients travel with it through the xylem. The cohesion-tension theory explains how leaves pull water through the xylem. Water molecules stick together, or exhibit cohesion. As a water molecule evaporates from the surface of the leaf, it pulls on the adjacent water molecule, creating a continuous flow of water through the plant.
Hydraulic Conductivity is a property of vascular plants, soils and rocks, that describes the ease with which a fluid (usually water) can move through pore spaces or fractures. It depends on the intrinsic permeability of the material, the degree of saturation, and on the density and viscosity of the fluid. Saturated hydraulic conductivity, Ksat, describes water movement through saturated media. By definition, hydraulic conductivity is the ratio of velocity to hydraulic gradient indicating permeability of porous media. Vascular Plants are defined as those land plants that have lignified tissues (the xylem) for conducting water and minerals throughout the plant. Phloem is the living tissue that transports the soluble organic compounds made during photosynthesis and known as photosynthates, in particular the sugar sucrose, to parts of the plant where needed. This transport process is called translocation.
Deuterium-Depleted Water is water which has a lower concentration of deuterium than occurs naturally. Deuterium is a heavier isotope of hydrogen which has, in addition to its one proton, a neutron that roughly doubles the mass of the hydrogen atom. In Vienna Standard Mean Ocean Water, deuterium occurs at a rate of 155.76 ppm. The production of heavy water involves isolating and removing deuterium within water. The by-product of this process is deuterium-depleted water.
Relative Permittivity of a material is its (absolute) permittivity expressed as a ratio relative to the permittivity of vacuum. Permittivity is a material property that affects the Coulomb force between two point charges in the material. Relative permittivity is the factor by which the electric field between the charges is decreased relative to vacuum. Likewise, relative permittivity is the ratio of the capacitance of a capacitor using that material as a dielectric, compared with a similar capacitor that has vacuum as its dielectric. Relative permittivity is also commonly known as dielectric constant, a term deprecated in physics and engineering as well as in chemistry.
Siphoning
Siphon is used to refer to a wide variety of devices that involve the flow of liquids through tubes. In a narrower sense, the word refers particularly to a tube in an inverted 'U' shape, which causes a liquid to flow upward, above the surface of a reservoir, with no pump, but powered by the fall of the liquid as it flows down the tube under the pull of gravity, then discharging at a level lower than the surface of the reservoir from which it came. At sea level, water can be lifted a little more than 10 metres or 33 feet. Pythagorean Cup is when a specially designed cup is filled beyond a certain point, a siphoning effect causes the cup to drain its entire contents through the base.
Air Lock - Hydraulic Pump - How To Make a Self-Starting Siphon (youtube)
Superfluidity is a state of matter in which the matter behaves like a fluid with zero viscosity; where it appears to exhibit the ability to self-propel and travel in a way that defies the forces of gravity and surface tension. Superfluidity is found in astrophysics, high-energy physics, and theories of quantum gravity. The phenomenon is related to Bose–Einstein condensation, but neither is a specific type of the other: not all Bose-Einstein condensates can be regarded as superfluids, and not all superfluids are Bose–Einstein condensates.
Atomization is the making of an aerosol, which is a colloid suspension of fine solid particles or liquid droplets in a gas. Sprays, mists, fogs, clouds, dust clouds, and smoke, which appear to be atomized. Atomizer Nozzle (wiki)
Aerosol is a suspension of fine solid particles or liquid droplets, in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog, dust, forest exudates and geyser steam. Examples of anthropogenic aerosols are haze, particulate air pollutants and smoke.
Aspirator pump is a type of ejector-jet pump, which produces vacuum by means of the Venturi effect, which is the reduction in fluid pressure that results when a fluid flows through a constricted section (or choke) of a pipe.
Nebulizer is a drug delivery device used to administer medication in the form of a mist inhaled into the lungs. Nebulizers are commonly used for the treatment of asthma, cystic fibrosis, COPD and other respiratory diseases or disorders.
Injector is typically used to deliver cold water to a boiler against its own pressure using its own live or exhaust steam, replacing any mechanical pump.
Xylem is one of the two types of transport tissue in vascular plants, phloem being the other. The basic function of xylem is to transport water from roots to stems and leaves, but it also transports nutrients. The word "xylem" is derived from the Greek word (xylon), meaning "wood"; the best-known xylem tissue is wood, though it is found throughout a plant. Water is a polar molecule. When two water molecules approach one another, the slightly negatively charged oxygen atom of one forms a hydrogen bond with a slightly positively charged hydrogen atom in the other. This attractive force, along with other intermolecular forces, is one of the principal factors responsible for the occurrence of surface tension in liquid water. It also allows plants to draw water from the root through the xylem to the leaf. Water is constantly lost through transpiration from the leaf. When one water molecule is lost another is pulled along by the processes of cohesion and tension. Transpiration pull, utilizing capillary action and the inherent surface tension of water, is the primary mechanism of water movement in plants. However, it is not the only mechanism involved. Any use of water in leaves forces water to move into them. Transpiration in leaves creates tension (differential pressure) in the cell walls of mesophyll cells. Because of this tension, water is being pulled up from the roots into the leaves, helped by cohesion (the pull between individual water molecules, due to hydrogen bonds) and adhesion (the stickiness between water molecules and the hydrophilic cell walls of plants). This mechanism of water flow works because of water potential (water flows from high to low potential), and the rules of simple diffusion
Viscosity
Viscosity fluid is a measure of its resistance to gradual deformation by shear stress or tensile stress. For liquids, it corresponds to the informal concept of "thickness"; for example, honey has a much higher viscosity than water. Heat.
Non-Newtonian Fluid turns into solids when pressure is applied. In non-Newtonian fluids, viscosity can change when under force to either more liquid or more solid. Ketchup, for example, becomes runnier when shaken and is thus a non-Newtonian fluid. Many salt solutions and molten polymers are non-Newtonian fluids, as are many commonly found substances such as custard, honey, toothpaste, starch suspensions, corn starch, paint, blood, and shampoo. This type of fluid does not follow Newton's law of viscosity, i.e., constant viscosity independent of stress. Surface Tension.
New study visualizes motion of water molecules, promises new wave of electronic devices. Studying the viscosity of water has revealed new insights about the behavior of water molecules and may open pathways for liquid-based electronics.
Cavitation is the formation of vapour cavities in a liquid, small liquid-free zones ("bubbles" or "voids"), that are the consequence of forces acting upon the liquid. It usually occurs when a liquid is subjected to rapid changes of pressure that cause the formation of cavities in the liquid where the pressure is relatively low. When subjected to higher pressure, the voids implode and can generate an intense shock wave. Cavitation is a significant cause of wear in some engineering contexts. Collapsing voids that implode near to a metal surface cause cyclic stress through repeated implosion. This results in surface fatigue of the metal causing a type of wear also called "cavitation". The most common examples of this kind of wear are to pump impellers, and bends where a sudden change in the direction of liquid occurs. Cavitation is usually divided into two classes of behavior: inertial (or transient) cavitation and non-inertial cavitation. Inertial cavitation is the process where a void or bubble in a liquid rapidly collapses, producing a shock wave. Inertial cavitation occurs in nature in the strikes of mantis shrimps and pistol shrimps, as well as in the vascular tissues of plants. In man-made objects, it can occur in control valves, pumps, propellers and impellers. Non-inertial cavitation is the process in which a bubble in a fluid is forced to oscillate in size or shape due to some form of energy input, such as an acoustic field. Such cavitation is often employed in ultrasonic cleaning baths and can also be observed in pumps, propellers, etc. Since the shock waves formed by collapse of the voids are strong enough to cause significant damage to moving parts, cavitation is usually an undesirable phenomenon. It is very often specifically avoided in the design of machines such as turbines or propellers, and eliminating cavitation is a major field in the study of fluid dynamics. However, it is sometimes useful and does not cause damage when the bubbles collapse away from machinery, such as in supercavitation, which is the use of cavitation effects to create a bubble of steam inside a liquid large enough to encompass an object travelling through the liquid, greatly reducing the skin friction drag on the object and enabling achievement of very high speeds. Cavitation produces a bubble that rapidly collapses and becomes hotter than the sun's surface. Cavitation is when low pressure in a liquid produces a bubble that rapidly collapses, and heats up to 20,000 Kelvin — hotter than the sun's surface. This usually releases a flash of light called sonoluminescence, which physicists still don't understand. Some physicists even theorize that cavitation bubbles could get hot enough to power nuclear fusion. Cavitation is the formation of an empty space within a solid object or body. The formation of bubbles in a liquid, typically by the movement of a propeller through it. Hydrodynamic cavitation describes the process of vaporisation, bubble generation and bubble implosion which occurs in a flowing liquid as a result of a decrease and subsequent increase in local pressure. ultrasound cavitation, a method of generating cavitation with soundwaves most well known for its use in breaking up kidney stones. Contraction - Impeller.
Dispersion in water waves generally refers to frequency dispersion, which means that waves of different wavelengths travel at different phase speeds. Water waves, in this context, are waves propagating on the water surface, with gravity and surface tension as the restoring forces. As a result, water with a free surface is generally considered to be a dispersive medium.
Acoustic Dispersion is the phenomenon of a sound wave separating into its component frequencies as it passes through a material. The phase velocity of the sound wave is viewed as a function of frequency. Hence, separation of component frequencies is measured by the rate of change in phase velocities as the radiated waves pass through a given medium.
Thickened Fluids have several levels of consistency/viscosity. 0 – Thin liquids: Unthickened, such as water or juice. Common thin liquids include coffee, tea, clear broth, clear juice, skim milk, 2% milk, and whole milk. 1 – Slightly thick (between 9 and 6 ml pour out of a 10ml syringe in 10 seconds). 2 – Mildly thick (between 6 and 2 ml pour out). 3 – Moderately thick (2 or less ml pour out). 4 – Extremely thick – drinks of this stage should require a spoon to drink and are comparable to pureed foods. People with dysphagia have difficulty in swallowing. Thicker consistency makes it less likely that individuals will aspirate while they are drinking. Pulmonary Aspiration is the entry of material such as pharyngeal secretions, food or drink, or stomach contents from the oropharynx or gastrointestinal tract, into the larynx (voice box) and lower respiratory tract, the portions of the respiratory system from the trachea (windpipe) to the lungs. A person may inhale the material, or it may be delivered into the tracheobronchial tree during positive pressure ventilation. When pulmonary aspiration occurs during eating and drinking, the aspirated material is often colloquially referred to as "going down the wrong pipe."
Heat - High Temperature
 When heat is added to a substance, the molecules and
atoms vibrate faster.
As atoms vibrate faster, the space between atoms increases. An increase in
the speed of the molecules competes with the attraction between molecules
and causes molecules to move a little further apart. The volume of a
gas increases more than the volume of a
solid or liquid. The end result of increased molecular motion is that the
object expands
and takes up more space.
Mass of the object remains the same,
however. Solids, liquids and gases all expand when heat is added. The
mass is not
changed.
When heat is added to a substance, the molecules and
atoms vibrate faster.
As atoms vibrate faster, the space between atoms increases. An increase in
the speed of the molecules competes with the attraction between molecules
and causes molecules to move a little further apart. The volume of a
gas increases more than the volume of a
solid or liquid. The end result of increased molecular motion is that the
object expands
and takes up more space.
Mass of the object remains the same,
however. Solids, liquids and gases all expand when heat is added. The
mass is not
changed.Thermal Expansion is the tendency of matter to change its shape, area, and volume in response to a change in temperature.
Temperature is a monotonic function of the average molecular kinetic energy of a substance. When a substance is heated, the kinetic energy of its molecules increases. Thus, the molecules begin vibrating/moving more and usually maintain a greater average separation. Materials which contract with increasing temperature are unusual; this effect is limited in size, and only occurs within limited temperature ranges. The relative expansion (also called strain) divided by the change in temperature is called the material's coefficient of thermal expansion and generally varies with temperature.
At low temperature a gas molecule travels, on the average, at a slower speed than than it would at a high temperature. When heat leaves all substances, the molecules vibrate slower. The atoms can get closer which results in the matter contracting. So, at a low temperature the molecules have, on the average, less kinetic energy than they do at a high temperature. Lower speeds, lower kinetic Energies. Cooling a liquid decreases the speed of the molecules. A decrease in the speed of the molecules allows the attractions between molecules to bring them a little closer together. The motion and spacing of the particles determines the state of matter of the substance.
Cooking Heat - Boiling Water - Evaporation - Potential Energy - Steam
Marangoni Effect is the mass transfer along an interface between two fluids due to a gradient of the surface tension. In the case of temperature dependence, this phenomenon may be called thermo-capillary convection (or Bénard–Marangoni convection).
Convection is the transfer of heat due to the bulk movement of molecules within fluids (gases and liquids), including molten rock (rheid). Convection includes sub-mechanisms of advection (directional bulk-flow transfer of heat), and diffusion (non-directional transfer of energy or mass particles along a concentration gradient).
Advection is the transport of a substance or quantity by bulk motion. The properties of that substance are carried with it. Generally the majority of the advected substance is a fluid. The properties that are carried with the advected substance are conserved properties such as energy.
Thermal Conduction is the transfer of internal energy by microscopic collisions of particles and movement of electrons within a body. The colliding particles, which include molecules, atoms and electrons, transfer disorganized microscopic kinetic and potential energy, jointly known as internal energy. Conduction takes place in all phases: solid, liquid, and gas.
Density of a substance is its mass per unit volume.
Why does Hot Air Rise? When an object or substance heats up, the atoms which compose it start to move and vibrate more quickly. This vibration pushes them apart, causing them to take up more space. That means that a given volume of air (or water, or whatever) will have fewer atoms in it, because some of them were pushed out as it expanded. Fewer atoms means less mass, means less weight per volume. Less weight per volume means less density. Thermals - Hot Water.
 Sky
Lantern is a small hot air balloon made of paper, with an opening at
the bottom where a small fire is suspended. They can be dangerous because
they could land and start a fire.
Sky
Lantern is a small hot air balloon made of paper, with an opening at
the bottom where a small fire is suspended. They can be dangerous because
they could land and start a fire.Hot Air Balloon is a lighter than air aircraft consisting of a bag, called an envelope, which contains heated air. Suspended beneath is a gondola or wicker basket (in some long-distance or high-altitude balloons, a capsule), which carries passengers and (usually) a source of heat, in most cases an open flame. The heated air inside the envelope makes it buoyant since it has a lower density than the colder air outside the envelope. As with all aircraft, hot air balloons cannot fly beyond the atmosphere. Unlike gas balloons, the envelope does not have to be sealed at the bottom, since the air near the bottom of the envelope is at the same pressure as the surrounding air. In modern sport balloons the envelope is generally made from nylon fabric and the inlet of the balloon (closest to the burner flame) is made from a fire resistant material such as Nomex. Modern balloons have been made in all kinds of shapes, such as rocket ships and the shapes of various commercial products, though the traditional shape is used for most non-commercial, and many commercial, applications. Wind Chill. Water Gas is produced from synthesis gas, which is composed of carbon monoxide and hydrogen. Syngas is a useful product but requires careful handling due to its flammability and the risk of carbon monoxide poisoning. The Water gas shift reaction can be used to reduce the carbon monoxide while producing additional hydrogen, resulting in Water Gas. Thermodynamics.
Why does Heating Water make it a better Solvent
Adding energy or heat increases molecular motion. Increased molecular motion competes with the attraction between solute molecules and tends to make them come apart more easily. Increased molecular motion causes more solvent molecules to contact solute molecules and pull on them with more force, usually resulting in more dissolving. Since different substances are made from different atoms, ions, or molecules, increased temperature will affect their dissolving to different extents. Hot Air.
Showers - Cooking Food
Viscosity - Evaporation - Boiling Water
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature, pressure and the pH of the solution. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration, where adding more solute does not increase the concentration of the solution and begins to precipitate the excess amount of solute. The solubility of a substance is an entirely different property from the rate of solution, which is how fast it dissolves. Solvents composed of polar molecules, such as water, dissolve other polar molecules, such as table salt, while nonpolar solvents, such as gasoline, dissolve nonpolar substances such as wax. The degree that a solvent dissolves a given solute is known as its solubility. Solute molecule is a molecule that's soluble.
"The probability of you drinking a glass of water that contains a molecule of water that also passed through a dinosaur is almost 100%."
Boiling Water at High Altitudes
Boiling Point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure.
Boiling is the application of heat to change something from a liquid to a gas. Cooking in a liquid that has been brought to a boil. Come to the boiling point and change from a liquid to vapor. Boiler.
High Altitude Cooking: A common misconception is that it takes longer to boil water at high altitudes. As explained, it is the exact opposite. Increased elevation = decreased boiling point. Thus this lower boiling point actually takes less time to reach, so water starts to boil at a lower temperature. The confusion is created by the fact that because of this lower boiling point, it actually takes longer to cook food in or over water. Because less Heat is being transferred through radiation, conduction, and convection, it will take longer to cook a pot of Alpine Pasta at 10,000 feet than at 1,000 feet elevation. Other factors not related to elevation gain, such as colder temperatures and windy conditions, can also increase the time required to cook food over a stove. These factors can be combated using a backpacking stove windscreen. Altitude affects cooking in three different ways: As elevation increases, the boiling point of water decreases – When water boils at lower temperatures, it takes longer for foods to cook in or over water because less heat is being transferred. As water’s boiling point decreases and cooking times increase, the quicker liquid will evaporate – Because water is boiling at a lower temperature, water will begin to evaporate sooner. As elevation increases, air pressure decreases and the faster leavening gases (air, carbon dioxide and water vapor) expand – This mostly only affects baking at high altitudes where the amount of leavening agents should be reduced. For water to increase in temperature, water molecules must be made to move faster within the water; this requires breaking hydrogen bonds, and the breaking of hydrogen bonds absorbs heat. Heat Capacity is the capability of water to absorb heat without undergoing an increase in temperature.
Water boils at 100 °C (212 °F) at sea level, but at 93.4 °C (200.1 °F) at 1,905 metres (6,250 ft) altitude. For a given pressure, different liquids will boil at different temperatures. Hot Showers.
Cooking with Hot Water - Stoves - Temperatures - Hot Air
Boil Water with No Heat! - Hydrostatics (youtube)
Mpemba Effect is the observation that, in some circumstances, warmer water can freeze faster than colder water. there is disagreement on exactly what the effect is and under what circumstances it occurs.
Leavening Agent (yeast fungi)
Fluid Mechanics is the branch of physics that studies the mechanics of fluids (liquids, gases, and plasmas) and the forces on them.
Hydrostatics is the branch of fluid mechanics that studies incompressible fluids at rest.
Bernoulli's Principle states that an increase in the speed of a fluid occurs simultaneously with a decrease in pressure or a decrease in the fluid's potential energy.
Pressure is the Force applied perpendicular to the surface of an object per unit area over which that force is distributed force divided by area.
Rapid decompression key to making low-density liquid water. There are at least 17 forms of water ice, and two proposed forms of super-cooled liquid water. New work from high-pressure geophysicists finds evidence of the long-theorized, difficult-to-see low-density liquid phase of water.
Barometer is a scientific instrument used in meteorology to measure atmospheric pressure.
Meteorology is the interdisciplinary scientific study of the atmosphere.
Atmospheric Pressure sometimes also called barometric pressure, is the pressure exerted by the weight of air in the atmosphere of Earth atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point.
Vapor Pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure. The vapor pressure of any substance increases non-linearly with temperature according to the Clausius–Clapeyron relation. The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapor bubbles inside the bulk of the substance. Bubble formation deeper in the liquid requires a higher temperature due to the higher fluid pressure, because fluid pressure increases above the atmospheric pressure as the depth increases. More important at shallow depths is the higher temperature required to start bubble formation. The surface tension of the bubble wall leads to an overpressure in the very small, initial bubbles. Thus, thermometer calibration should not rely on the temperature in boiling water. The vapor pressure that a single component in a mixture contributes to the total pressure in the system is called partial pressure. For example, air at sea level, and saturated with water vapor at 20 °C, has partial pressures of about 2.3 kPa of water, 78 kPa of nitrogen, 21 kPa of oxygen and 0.9 kPa of argon, totaling 102.2 kPa, making the basis for standard atmospheric pressure. Water Vapor.
Microwave Ovens essentially heat up the water content of the food. If the food has a skin or sealed layer, like potatoes, then pressure builds up on the inside as trapped moisture turns into steam. As the pressure exceeds the strength of the skin the food “bursts” with an explosive release of steam. In order to reduce the odds of food exploding in your microwave, you want to give the steam a place to escape. Simply take a fork and pierce the food item several times, Snider suggests. It's the same technique you've been using all along before heating those frozen dinners. Properties such as such as shape and size, are affected by oven power and food placement during microwave heating.
The origin of water’s unusual properties found. Using x-ray lasers, researchers at Stockholm University have been able to map out how water fluctuates between two different states when it is cooled. At -44°C these fluctuations reach a maximum pointing to the fact that water can exist as two different distinct liquids. How water’s density, specific heat, viscosity and compressibility respond to changes in pressure and temperature is completely opposite to other liquids that we know. We all are aware that all matter shrinks when it is cooled resulting in an increase in the density. We would therefore expect that water would have high density at the freezing point. However, if we look at a glass of ice water, everything is upside down, since we expect that water at 0°C being surrounded by ice should be at the bottom of the glass, but of course as we know ice cubes float. Strangely enough for the liquid state, water is the densest at 4 degrees C, and therefore it stays on the bottom whether it’s in a glass or in an ocean. If you chill water below 4 degrees, it starts to expand again. If you continue to cool pure water (where the rate of crystallization is low) to below 0, it continues to expand – the expansion even speeds up when it gets colder. Many more properties such as compressibility and heat capacity become increasingly strange as water is cooled. Now researchers at Stockholm University, with the help of ultra-short x-ray pulses at x-ray lasers in Japan and South Korea, have succeeded in determining that water reaches the peak of its strange behaviour at -44°C. Water is unique, as it can exist in two liquid states that have different ways of bonding the water molecules together. The water fluctuates between these states as if it can’t make up its mind and these fluctuations reach a maximum at -44°C. It is this ability to shift from one liquid state into another that gives water its unusual properties and since the fluctuations increase upon cooling also the strangeness increases.
Leidenfrost Effect is a physical phenomenon in which a liquid, close to a surface that is significantly hotter than the liquid's boiling point, produces an insulating vapor layer that keeps the liquid from boiling rapidly. Because of this 'repulsive force', a droplet hovers over the surface rather than making physical contact with the hot surface. This is most commonly seen when cooking, when a few drops of water are sprinkled in a hot pan. If the pan's temperature is at or above the Leidenfrost point, which is approximately 193 °C (379 °F) for water, the water skitters across the pan and takes longer to evaporate than it would take if the water droplets had been sprinkled into a cooler pan. The effect is responsible for the ability of a person to quickly dip a wet finger in molten lead or blow out a mouthful of liquid nitrogen without injury. The latter is potentially lethal, particularly should one accidentally swallow the liquid nitrogen.
Ice - Snow - Crystals
Ice is water frozen into a solid state. Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color. Temperature.
Ice Crystals are solid ice exhibiting atomic ordering on various length scales and include hexagonal columns, hexagonal plates, dendritic crystals, and diamond dust. The highly symmetric shapes are due to depositional growth, namely, direct deposition of water vapour onto the ice crystal. Freezing Food - Freezing Humans - Hibernation.
Ice Ih ice-phase-one is the hexagonal crystal form of ordinary ice, or frozen water. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic that is occasionally present in the upper atmosphere. Ice Ih exhibits many peculiar properties that are relevant to the existence of life and regulation of global climate.
Ice Ic is a metastable cubic crystalline variant of ice.
Ice II is a rhombohedral crystalline form of ice with a highly ordered structure. It is formed from ice Ih by compressing it at temperature of 198 K at 300 MPa or by decompressing ice V. When heated it undergoes transformation to ice III. Ordinary water ice is known as ice Ih.
Ice III is a form of solid matter which consists of tetragonal crystalline ice, formed by cooling water down to 250 K at 300 MPa. It is the least dense of the high-pressure water phases, with a density of 1160 kg/m3 (at 350 MPa). The proton-ordered form of ice III is ice IX. Water that Never Freezes can reach minus 263 degrees Celsius without turning into ice.
Ice Cutting Experiment (youtube)
Supercooling is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid.
Supercritical Liquid-Gas Boundaries are lines in the pressure-temperature (pT) diagram that delimit more liquid-like and more gas-like states of a supercritical fluid. They comprise the Fisher–Widom line, the Widom line, and the Frenkel line.
Regelation is the phenomenon of melting under pressure and freezing again when the pressure is reduced. Many sources state that regelation can be demonstrated by looping a fine wire around a block of ice, with a heavy weight attached to it.
De-icing is the process of removing snow, ice or frost from a surface. Anti-icing is understood to be the application of chemicals that not only de-ice but also remain on a surface and continue to delay the reformation of ice for a certain period of time, or prevent adhesion of ice to make mechanical removal easier. Ice-proof coating for big structures.
Cold is having a low or inadequate temperature. The feeling or sensation of coldness or having been made cold by ice or refrigeration. The sensation produced by low temperatures. Lacking the warmth of life or the absence of heat.
Spinning ice disk in Michigan's Pine River. An ice disc forms when a section of ice on a partially frozen river breaks off and is pushed in circular rotation by an eddy current, smoothing the ice disc into a perfect circle.
Dunking a steel ball into liquid nitrogen and then into water. Water freezing is an exothermic process. Water heats up as it freezes. -321°F Japanese Mirror-Polished Ice Ball— Exothermic Ice Formation (youtube).
Exothermic Process describes a process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen).
Evaporation - Dehydration
Halo as an optical phenomenon is an optical phenomenon produced by Light interacting with ice crystals suspended in the atmosphere, resulting in a wide variety of colored or white rings, arcs and spots in the sky. Many halos are near the Sun or Moon, but others occur elsewhere or even in the opposite part of the sky. Halo types are the circular halo (properly called the 22° halo), light pillars and sun dogs, but there are many more; some of them fairly common, others (extremely) rare. (Halo is also known as a nimbus, icebow or gloriole).
Rainbows - Holography - Aurora.
Vapor Tracers light up as they interact with ionized or neutral particles in the atmosphere, making the movements of these particles visible. Different types of tracers light up in the presence of different particles. When barium is exposed to sunlight it ionizes rapidly and glows purple-red. Watching the dance of the barium clouds could provide information about how charged particles move in the ionosphere. But barium that is not ionized, which can be enhanced with the addition of strontium or lithium, can also be used to track neutral particles. Lithium alone can also be used to track neutral winds and can actually be used during daylight to track emissions, but glows a bright red at night.
Ice Core Laboratory: Analizing Earths History by studying Ice Cores that go back thousands of years.
Ice Water Molecules Arranged in a Lattice of Squares Between 2 Sheets of Graphene (youtube)
Frogs that avoid freezing to death when frozen solid. North American Frog, Marsh Frog and Wood Frog.
Ringwoodite is a high-pressure phase of Mg2SiO4 formed at high temperatures and pressures of the Earth's mantle between 525 and 660 km (326 and 410 mi) depth. It is polymorphous with the olivine phase forsterite (a magnesium iron silicate). Ringwoodite is notable for being able to contain hydroxide ions (oxygen and hydrogen atoms bound together) within its structure.
Wadsleyite is a high-pressure phase of polymorphous Mg2SiO4. An orthorhombic mineral with the formula β-Mg2SiO4, it was first found in nature in the Peace River meteorite from Alberta, Canada. It is formed by a phase transformation from forsterite (α-Mg2SiO4) under increasing pressure and eventually transforms into spinel-structured ringwoodite (γ-Mg2SiO4) as pressure increases further. The structure can take up a limited amount of other bivalent cations instead of magnesium, but contrary to the α and γ structures, a β structure with the sum formula Fe2SiO4 is not thermodynamically stable. Its cell parameters are approximately a = 5.7 Å, b = 11.7 Å and c = 8.24 Å.
Ice Cap is an ice mass that covers less than 50,000 km2 of land area (usually covering a highland area). Larger ice masses covering more than 50,000 km2 are termed ice sheets. Ice caps are not constrained by topographical features (i.e., they will lie over the top of mountains). By contrast, ice masses of similar size that are constrained by topographical features are known as ice fields. The dome of an ice cap is usually centred on the highest point of a massif. Ice flows away from this high point (the ice divide) towards the ice cap's periphery. Ice caps have significant effects on the geomorphology of the area they occupy. Plastic moulding, gouging and other glacial erosional features become present upon the glacier's retreat. Many lakes, such as the Great Lakes in North America, as well as numerous valleys have been formed by glacial action over hundreds of thousands of years. On Earth, there are about 30 million km3 of total ice mass. The average temperature of an ice mass ranges between −20 °C and −30 °C. The core of an ice cap exhibits a constant temperature that ranges between −15 °C and −20 °C. A high-latitude region covered in ice, though strictly not an ice cap (since they exceed the maximum area specified in the definition above), are called polar ice caps; the usage of this designation is widespread in the mass media and arguably recognized by experts. Vatnajökull is an example of an ice cap in Iceland.
Accelerated Warming from Albedo. Lost sea ice exposes dark, open waters, dramatically shifting the ocean surface from highly reflective to one that absorbs most of the sun's energy. This can set off a vicious cycle: ice loss leads to further warming of the ocean surface, which can lead to more ice loss. The loss of polar reflectivity (albedo) is one climate-related amplification that has scientists losing sleep at night. Accelerated warming due to higher Arctic temperatures. The loss and thinning of Arctic sea ice raises regional temperatures, delaying the formation of sea ice in the fall, and transferring more heat from the ocean to the air. If higher air temperatures speed the degradation of frozen ground (permafrost) on adjacent lands, they could release vast stores of carbon often trapped in the permafrost for thousands of years—further amplifying climate change.
Albedo is the measure of the diffuse reflection of solar radiation out of the total solar radiation received by an astronomical body (e.g. a planet like Earth). It is dimensionless and measured (via an albedometer) on a scale from 0 (corresponding to a black body that absorbs all incident radiation) to 1 (corresponding to a body that reflects all incident radiation). Surface albedo is defined as the ratio of radiosity to the irradiance (flux per unit area) received by a surface. The proportion reflected is not only determined by properties of the surface itself, but also by the spectral and angular distribution of solar radiation reaching the Earth's surface. These factors vary with atmospheric composition, geographic location and time (see position of the Sun). While bi-hemispherical reflectance is calculated for a single angle of incidence (i.e., for a given position of the Sun), albedo is the directional integration of reflectance over all solar angles in a given period. The temporal resolution may range from seconds (as obtained from flux measurements) to daily, monthly, or annual averages. Unless given for a specific wavelength (spectral albedo), albedo refers to the entire spectrum of solar radiation. Due to measurement constraints, it is often given for the spectrum in which most solar energy reaches the surface (between 0.3 and 3 μm). This spectrum includes visible light (0.4–0.7 μm), which explains why surfaces with a low albedo appear dark (e.g., trees absorb most radiation), whereas surfaces with a high albedo appear bright (e.g., snow reflects most radiation). Albedo is an important concept in climatology, astronomy, and environmental management (e.g., as part of the Leadership in Energy and Environmental Design (LEED) program for sustainable rating of buildings). The average albedo of the Earth from the upper atmosphere, its planetary albedo, is 30–35% because of cloud cover, but widely varies locally across the surface because of different geological and environmental features.
Glacier is a persistent body of dense ice that is constantly moving under its own weight; it forms where the accumulation of snow exceeds its ablation (melting and sublimation) over many years, often centuries. Glaciers slowly deform and flow due to stresses induced by their weight, creating crevasses, seracs, and other distinguishing features. They also abrade rock and debris from their substrate to create landforms such as cirques and moraines. Glaciers form only on land and are distinct from the much thinner sea ice and lake ice that form on the surface of bodies of water. On Earth, 99% of glacial ice is contained within vast ice sheets in the polar regions, but glaciers may be found in mountain ranges on every continent including Oceania's high-latitude oceanic islands such as New Zealand and Papua New Guinea. Between 35°N and 35°S, glaciers occur only in the Himalayas, Andes, Rocky Mountains, a few high mountains in East Africa, Mexico, New Guinea and on Zard Kuh in Iran. Glaciers cover about 10 percent of Earth's land surface. Continental glaciers cover nearly 13,000,000 km2 (5×106 sq mi) or about 98 percent of Antarctica's 13,200,000 km2 (5.1×106 sq mi), with an average thickness of 2,100 m (7,000 ft). Greenland and Patagonia also have huge expanses of continental glaciers. Glacial ice is the largest reservoir of fresh water on Earth. Many glaciers from temperate, alpine and seasonal polar climates store water as ice during the colder seasons and release it later in the form of meltwater as warmer summer temperatures cause the glacier to melt, creating a water source that is especially important for plants, animals and human uses when other sources may be scant. Within high-altitude and Antarctic environments, the seasonal temperature difference is often not sufficient to release meltwater. Because glacial mass is affected by long-term climatic changes, e.g., precipitation, mean temperature, and cloud cover, glacial mass changes are considered among the most sensitive indicators of climate change and are a major source of variations in sea level. A large piece of compressed ice, or a glacier, appears blue, as large quantities of water appear blue. This is because water molecules absorb other colors more efficiently than blue. The other reason for the blue color of glaciers is the lack of air bubbles. Air bubbles, which give a white color to ice, are squeezed out by pressure increasing the density of the created ice.
Rain - Erosion
Beautiful Glaciers and Ice Photos
Some of Earth’s water may have existed before the Sun was born?
Deuterium is one of two stable isotopes of hydrogen. The nucleus of deuterium, called a deuteron, contains one proton and one neutron, whereas the far more common hydrogen isotope, protium, has no neutron in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom in 6420 of hydrogen.
Snow
 Snowflakes is a single ice crystal that has achieved a
sufficient size, and may have amalgamated with others, then falls through
the Earth's atmosphere as snow. Each flake nucleates around a dust
particle in supersaturated air masses by attracting supercooled cloud
water droplets, which freeze and accrete in crystal form.
Complex Shapes
emerge as the flake moves through differing temperature and
humidity zones in the atmosphere, such that
individual snowflakes differ in detail from one another, but may be
categorized in eight broad classifications and at least 80 individual
variants. The main constituent shapes for ice crystals, from which
combinations may occur, are needle, column, plate and rime. Snowflakes
appear white in color despite being made of clear ice. This is due to
diffuse reflection of the whole spectrum of light by the small crystal
facets. Once snowflakes land and accumulate, they undergo metamorphosis
with changes in temperature and coalesce into a snowpack. The
characteristics of the snowpack reflect the changed nature of the
constituent snow crystals. Snow is the lightest form of
water. A snow flake is 0.02 grams and it takes 50 flakes to make a
gram.
Snowflakes is a single ice crystal that has achieved a
sufficient size, and may have amalgamated with others, then falls through
the Earth's atmosphere as snow. Each flake nucleates around a dust
particle in supersaturated air masses by attracting supercooled cloud
water droplets, which freeze and accrete in crystal form.
Complex Shapes
emerge as the flake moves through differing temperature and
humidity zones in the atmosphere, such that
individual snowflakes differ in detail from one another, but may be
categorized in eight broad classifications and at least 80 individual
variants. The main constituent shapes for ice crystals, from which
combinations may occur, are needle, column, plate and rime. Snowflakes
appear white in color despite being made of clear ice. This is due to
diffuse reflection of the whole spectrum of light by the small crystal
facets. Once snowflakes land and accumulate, they undergo metamorphosis
with changes in temperature and coalesce into a snowpack. The
characteristics of the snowpack reflect the changed nature of the
constituent snow crystals. Snow is the lightest form of
water. A snow flake is 0.02 grams and it takes 50 flakes to make a
gram.What does a snowflake look like in zero gravity?
Ice Pellets are a form of precipitation consisting of small, translucent balls of ice. Ice pellets are smaller than hailstones which form in thunderstorms rather than in winter, and are different from graupel ("soft hail") which is made of frosty white rime, and from a mixture of rain and snow which is a slushy liquid or semisolid. Ice pellets often bounce when they hit the ground or other solid objects, and make a higher-pitched "tap" when striking objects like jackets, windshields, and dried leaves, compared to the dull splat of liquid raindrops. Pellets generally do not freeze into a solid mass unless mixed with freezing rain. The METAR code for ice pellets is PL (PE before November 1998). Ice Storm (wiki).
Hail is a form of solid precipitation. It is distinct from ice pellets (American English "Sleet"), though the two are often confused. It consists of balls or irregular lumps of ice, each of which is called a hailstone. Hail is layers of ice. Ice pellets fall generally in cold weather while hail growth is greatly inhibited during cold surface temperatures. Unlike other forms of water ice such as graupel, which is made of rime, and ice pellets, which are smaller and translucent, hailstones usually measure between 5 mm (0.2 in) and 15 cm (6 in) in diameter. The METAR reporting code for hail 5 mm (0.20 in) or greater is GR, while smaller hailstones and graupel are coded GS. Hail is possible within most thunderstorms as it is produced by cumulonimbus, and within 2 nmi (3.7 km) of the parent storm. Hail formation requires environments of strong, upward motion of air with the parent thunderstorm (similar to tornadoes) and lowered heights of the freezing level. In the mid-latitudes, hail forms near the interiors of continents, while in the tropics, it tends to be confined to high elevations. There are methods available to detect hail-producing thunderstorms using weather satellites and weather radar imagery. Hailstones generally fall at higher speeds as they grow in size, though complicating factors such as melting, friction with air, wind, and interaction with rain and other hailstones can slow their descent through Earth's atmosphere. Severe weather warnings are issued for hail when the stones reach a damaging size, as it can cause serious damage to human-made structures and, most commonly, farmers' crops.
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point. For most substances, the melting and freezing points are the same temperature; however, certain substances possess differing solid–liquid transition temperatures.
Antifreeze Protein refers to a class of polypeptides produced by certain animals, plants, fungi and bacteria that permit their survival in temperatures below the freezing point of water. AFPs bind to small ice crystals to inhibit the growth and recrystallization of ice that would otherwise be fatal. There is also increasing evidence that AFPs interact with mammalian cell membranes to protect them from cold damage. This work suggests the involvement of AFPs in cold acclimatization. Antarctic fish have antifreeze blood, but it might fill them with ice crystals over time. In the icy waters of the Antarctic, most of the native fish have special proteins in their blood that act like antifreeze. The proteins bind to ice crystals, keeping them small to prevent the formation of fish popsicles.
Avalanche is an event that occurs when a cohesive slab of snow lying upon a weaker layer of snow fractures and slides down a steep slope. Avalanches are typically triggered in a starting zone from a mechanical failure in the snowpack (slab avalanche) when the forces of the snow exceed its strength but sometimes only with gradual widening (loose snow avalanche). After initiation, avalanches usually accelerate rapidly and grow in mass and volume as they entrain more snow. An avalanche can travel up to 4 times as fast as water over a waterfall. If the avalanche moves fast enough, some of the snow may mix with the air forming a powder snow avalanche, which is a type of gravity current. Slides of rocks or debris, behaving in a similar way to snow, are also referred to as avalanches (see rockslide). The remainder of this article refers to snow avalanches. The load on the snowpack may be only due to gravity, in which case failure may result either from weakening in the snowpack or increased load due to precipitation. Avalanches initiated by this process are known as spontaneous avalanches. Avalanches can also be triggered by other loading conditions such as human or biologically related activities. Seismic activity may also trigger the failure in the snowpack and avalanches. Although primarily composed of flowing snow and air, large avalanches have the capability to entrain ice, rocks, trees, and other surficial material. However, they are distinct from slushflows which have higher water content and more laminar flow, mudslides which have greater fluidity, rock slides which are often ice free, and serac collapses during an icefall. Avalanches are not rare or random events and are endemic to any mountain range that accumulates a standing snowpack. Avalanches are most common during winter or spring but glacier movements may cause ice and snow avalanches at any time of year. In mountainous terrain, avalanches are among the most serious objective natural hazards to life and property, with their destructive capability resulting from their potential to carry enormous masses of snow at high speeds. There is no universally accepted classification system for different forms of avalanches. Avalanches can be described by their size, their destructive potential, their initiation mechanism, their composition and their dynamics.
Crystals
 Crystal is a
solid material whose
composite parts are arranged in a
highly ordered microscopic
structure, forming a
crystal lattice that
extends in all directions. In addition, macroscopic single crystals are
usually identifiable by their geometrical shape, consisting of flat faces
with specific, characteristic orientations. The scientific study of
crystals and crystal formation is known as
crystallography. The process of crystal formation via mechanisms of
crystal growth is called
crystallization or solidification.(atoms,
molecules, or ions).
Crystal is a
solid material whose
composite parts are arranged in a
highly ordered microscopic
structure, forming a
crystal lattice that
extends in all directions. In addition, macroscopic single crystals are
usually identifiable by their geometrical shape, consisting of flat faces
with specific, characteristic orientations. The scientific study of
crystals and crystal formation is known as
crystallography. The process of crystal formation via mechanisms of
crystal growth is called
crystallization or solidification.(atoms,
molecules, or ions).
Rocks (minerals) - Fractals (symmetry) - Automaton.
Crystalline is consisting of crystals or composed of crystals or containing the nature of crystals. Having the structure and form of a crystal. Distinctly or sharply outlined. Transmitting light; able to be seen through with clarity.
Crystallography is the branch of science that studies the formation and structure of crystals. The experimental science of determining the arrangement of atoms in crystalline solids. Arrays.
Crystal Structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter.
Crystallization is the natural or artificial process where a solid forms where the atoms or molecules are highly organized in a structure known as a crystal. Some of the ways which crystals form are through precipitating from a solution, melting or more rarely deposition directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore related to precipitation, although the result is not amorphous or disordered, but a crystal.
What does Ice Crystals look like in Space?
Pattern Formation during Ice Crystal Growth?
Time Crystal is a structure that repeats in time, as well as in space. Normal three-dimensional crystals have a repeating pattern in space, but remain unchanged as time passes. Time crystals repeat themselves in time as well, leading the crystal to change from moment to moment. If a discrete time translation symmetry is broken (which may be realized in open driven systems), then the system is referred to as a discrete time crystal. A discrete time crystal never reaches thermal equilibrium, as it is a type of non-equilibrium matter, a form of matter proposed in 2012, and first observed in 2017.
Adatom is an atom that lies on a crystal surface, and can be thought of as the opposite of a surface vacancy, which is a type of point defect in a crystal.
Physics: Crystals 1958 Alan Holden - Bell Laboratories - PSSC Physical Science Study Committee (youtube) - Crystal is based on the microscopic arrangement of atoms inside it, called the crystal structure. A crystal is a solid where the atoms form a periodic arrangement. (Quasicrystals are an exception. Not all solids are crystals. Most macroscopic inorganic solids are polycrystalline, including almost all metals, ceramics, ice, rocks, etc. Solids that are neither crystalline nor polycrystalline, such as glass, are called amorphous solids, also called glassy, vitreous, or noncrystalline. These have no periodic order, even microscopically. There are distinct differences between crystalline solids and amorphous solids: most notably, the process of forming a glass does not release the latent heat of fusion, but forming a crystal does.
Crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions.
Texture in crystalline is the distribution of crystallographic orientations of a polycrystalline sample (it is also part of the geological fabric). A sample in which these orientations are fully random is said to have no distinct texture. If the crystallographic orientations are not random, but have some preferred orientation, then the sample has a weak, moderate or strong texture. The degree is dependent on the percentage of crystals having the preferred orientation. Texture is seen in almost all engineered materials, and can have a great influence on materials properties. Also, geologic rocks show texture due to their thermo-mechanic history of formation processes.
Alum any of a number of analogous crystalline double sulfates of a monovalent metal (or group) and a trivalent metal.
Mica group of sheet silicate (phyllosilicate) minerals includes several closely related materials having nearly perfect basal cleavage. All are monoclinic, with a tendency towards pseudohexagonal crystals, and are similar in chemical composition. The nearly perfect cleavage, which is the most prominent characteristic of mica, is explained by the hexagonal sheet-likearrangement of its atoms. Graphene.
Thousands of stars turning into crystals. The first direct evidence of white dwarf stars solidifying into crystals has been discovered by astronomers, and our skies are filled with them.
Amorphous Solid is a solid that lacks the long-range order that is characteristic of a crystal. Glass is an amorphous solid that exhibits a glass transition. Polymers are often amorphous. Other types of amorphous solids include gels, thin films, and nanostructured materials such as glass.
New crystalline form of ice. Scientists elucidate crystal structure for exotic ice XIX. Three years ago in 2018, chemists found evidence for the existence of a new variety of ice. Until then, 18 types of crystalline ice were known. The team now reports on the elucidation of the crystal structure of ice XIX using neutron diffraction.
New method to design diamond lattices and other crystals from microscopic building blocks. Researchers describe a technique for using LEGO®-like elements at the scale of a few billionths of a meter. Further, they are able to cajole these design elements to self-assemble, with each LEGO® piece identifying its proper mate and linking up in a precise sequence to complete the desired nanostructure.
Colloidal Crystal is an ordered array of colloid particles and fine grained materials analogous to a standard crystal whose repeating subunits are atoms or molecules. A natural example of this phenomenon can be found in the gem opal, where spheres of silica assume a close-packed locally periodic structure under moderate compression. Bulk properties of a colloidal crystal depend on composition, particle size, packing arrangement, and degree of regularity. Applications include photonics, materials processing, and the study of self-assembly and phase transitions. Colloidal Crystal Template - Nano Machines.
Dormant - Resurrection - Waiting for the Right Time
Resurrection Plant is any poikilohydric plant that can survive extreme dehydration, even over months or years. Longevity.
Synchronous and Asynchronous Communication - Seeds - Metamorphosis - Time Lapse
Back From the Brink. Biologists explore the molecular underpinnings of cells that recover from the verge of programmed death.
Scores of plant species are capable of Living Dormant under the soil for up to 20 years, enabling them to survive through difficult times. Rip Van Winkle (wiki).
Dormant is a condition of biological rest or suspended animation and inactive but capable of becoming active. A long geological life-cycle or very slow growing. Not erupting but not extinct either. Reemerge is to appear again.
Adaptation - Regeneration - Decay Half-Life - Entropy - Decompose - Cryogenics (frozen in time)
Dormancy is a period in an organism's life cycle when growth, development, and (in animals) physical activity are temporarily stopped. This minimizes metabolic activity and therefore helps an organism to conserve energy. Ice.
Hibernation is a state of inactivity and metabolic depression in endotherms. Hibernation refers to a season of heterothermy characterized by low body temperature, slow breathing and heart rate, and low metabolic rate. Grizzly bears' muscles don't atrophy during hibernation because they produce additional amino acids that stimulate muscle cell growth. Sleeping.
Suspended Animation is the temporary cessation of most vital functions without death, as in a dormant seed or a hibernating animal. Viruses.
Brumation is a term used for the hibernation-like state that cold-blooded animals utilize during very cold weather. On the other end of the spectrum is a state known as aestivation, which like brumation, provides a way for reptiles to handle temperature extremes. All reptiles, including snakes, lizards, turtles, tortoises, alligators, and crocodiles, some insects such as the busy dragonflies and bees, amphibians such as frogs, toads, and salamanders, as well as fish, including sharks, are all cold-blooded animals. Drought.
Plants adapted to Mediterranean climate may be more flexible in face of unpredictable rains.
Aestivation is a state of animal dormancy, similar to hibernation, characterized by inactivity and a lowered metabolic rate, that is entered in response to high temperatures and arid conditions. It takes place during times of heat and dryness, the hot dry season, which are often the summer months. Invertebrate and vertebrate animals are known to enter this state to avoid damage from high temperatures and the risk of desiccation. Both terrestrial and aquatic animals undergo aestivation. The fossil record suggests that aestivation may have evolved several hundred million years ago. Organisms that aestivate appear to be in a fairly "light" state of dormancy, as their physiological state can be rapidly reversed, and the organism can quickly return to a normal state. A study done on Otala lactea, a snail native to parts of Europe and Northern Africa, shows that they can wake from their dormant state within ten minutes of being introduced to a wetter environment. The primary physiological and biochemical concerns for an aestivating animal are to conserve energy, retain water in the body, ration the use of stored energy, handle the nitrogenous end products, and stabilize bodily organs, cells, and macromolecules. This can be quite a task as hot temperatures and arid conditions may last for months. The depression of metabolic rate during aestivation causes a reduction in macromolecule synthesis and degradation. To stabilize the macromolecules, aestivators will enhance antioxidant defenses and elevate chaperone proteins. This is a widely used strategy across all forms of hypometabolism. These physiological and biochemical concerns appear to be the core elements of hypometabolism throughout the animal kingdom. In other words, animals who aestivate appear to go through nearly the same physiological processes as animals that hibernate.
Cicadas spend most of their lives as underground nymphs, emerging only after 13 or 17 years, which may reduce losses by starving their predators and eventually emerging in huge numbers that overwhelm and satiate any remaining predators. The annual cicadas are species that emerge every year. Though these cicada have lifecycles that can vary from one to nine or more years as underground larvae, their emergence above ground as adults is not synchronized, so some appear every year.
Cicadas have prominent eyes set wide apart, short antennae, and membranous front wings. They have an exceptionally loud song, produced in most species by the rapid buckling and unbuckling of drumlike tymbals. Cicadidae has more than 3,000 species described from around the world; many species remain undescribed.
List of Longest-Living Organisms (wiki)
Dormouse can hibernate six months out of the year, or even longer if the weather does not become warm enough, sometimes waking for brief periods to eat food they had previously stored nearby. During the summer, they accumulate fat in their bodies to nourish them through the hibernation period. The sleepy behavior of The Dormouse character in Lewis Carroll's Alice's Adventures in Wonderland reflects this familiar trait of dormice.
Biological Immortality is a state in which the rate of mortality from senescence is stable or decreasing, thus decoupling it from chronological age. Various unicellular and multicellular species, including some vertebrates, achieve this state either throughout their existence or after living long enough. A biologically immortal living being can still die from means other than senescence, such as through injury or disease. A long geological life-cycle or very slow growing organism.
Potential Involvement of Snail Members in Neuronal Survival and Astrocytic Migration during the Gecko Spinal Cord Regeneration. - Regeneration (stem cells).
Rest Frame of a particle is the coordinate system (frame of reference) in which the particle is at Rest.
Seeds inherit memories from their mother. Maternal and environmental control of seed dormancy is carried out through novel epigenetic mechanisms. Seeds remain in a dormant state as long as environmental conditions are not ideal for germination. The depth of this sleep is inherited from their mother. Researchers reveal how this maternal imprint is transmitted through fragments of 'interfering' RNAs, which inactivate genes, and that a similar mechanism enables to transmit another imprint, that of the temperatures present during the development of the seed. This mechanism allows the seed to optimize the timing of its germination.
Vernalization is the induction of a plant's flowering process by exposure to the prolonged cold of winter, or by an artificial equivalent. After vernalization, plants have acquired the ability to flower, but they may require additional seasonal cues or weeks of growth before they will actually flower. This is sometimes used to refer to herbal (non-woody) plants requiring a cold dormancy to produce new shoots and leaves but this usage is discouraged. Many plants grown in temperate climates require vernalization and must experience a period of low winter temperature to initiate or accelerate the flowering process. This ensures that reproductive development and seed production occurs in spring and winters, rather than in autumn. The needed cold is often expressed in chill hours. Typical vernalization temperatures are between 5 and 10 degrees Celsius (40 and 50 degrees Fahrenheit). For many perennial plants, such as fruit tree species, a period of cold is needed first to induce dormancy and then later, after the requisite period of time, re-emerge from that dormancy prior to flowering. Many monocarpic winter annuals and biennials, including some ecotypes of Arabidopsis thaliana and winter cereals such as wheat, must go through a prolonged period of cold before flowering occurs. Flowering Locus C is a MADS-box gene that in late-flowering ecotypes of the plant Arabidopsis thaliana is responsible for vernalization. In a new seedling FLC is expressed, which prevents flowering. Upon exposure to cold, less FLC is expressed (to a degree depending on the amount of cold), and flowering becomes possible. FLC is extensively regulated through epigenetic modifications and transcriptional control. Plants and seeds can sense temperature changes, sunlight changes and moisture changes. Plants can communicate.
Poikilohydry is the lack of ability (structural or functional mechanism) to maintain and/or regulate water content to achieve homeostasis of cells and tissue connected with quick equilibration of cell/tissue water content to that of the environment. Frequently, it is coupled with the capacity to tolerate dehydration to low cell or tissue water content and to recover from it without physiological damage. This condition occurs in such organisms as the lichens and bryophytes that lack mechanisms, such as a waterproofing cuticle and stomata that can help to prevent desiccation. Poikilohydry is also noted in many forms of algae, which may be able to survive desiccation between successive high tides, or during occasional stranding due to the drying of a lake or pond. Similarly, poikilohydry occurs in land plants which survive environmental conditions when water supplies are seasonal or intermittent, as in the liverwort genus Targionia, which lives in Mediterranean habitats with hot dry summers.
Desiccation is the state of extreme dryness, or the process of extreme drying. A desiccant is a hygroscopic (attracts and holds water) substance that induces or sustains such a state in its local vicinity in a moderately sealed container.
Desiccator are sealable enclosures containing desiccants used for preserving moisture-sensitive items such as cobalt chloride paper for another use. A common use for desiccators is to protect chemicals which are hygroscopic or which react with water from humidity.
Activation of the NaCl- and drought-induced RD29A and RD29B promoters by constitutively active Arabidopsis MAPKK or MAPK proteins.
Eragrostis Nindensis
Transcriptome is the set of all messenger RNA molecules in one cell or a population of cells. It differs from the exome in that it includes only those RNA molecules found in a specified cell population, and usually includes the amount or concentration of each RNA molecule in addition to the molecular identities.
Proteome is the entire set of proteins expressed by a genome, cell, tissue, or organism at a certain time. More specifically, it is the set of expressed proteins in a given type of cell or organism, at a given time, under defined conditions. The term is a blend of proteins and genome. Proteomics is the study of the proteome.
Lipidome refers to the totality of lipids in cells. Lipids are one of the four major molecular components of biological organisms, along with proteins, sugars and nucleic acids.
Promoter in genetics is a region of DNA that initiates transcription of a particular gene. Promoters are located near the transcription start sites of genes, on the same strand and upstream on the DNA (towards the 5' region of the sense strand). Promoters can be about 100–1000 base pairs long.
Arabidopsis are small flowering plants related to cabbage and mustard. This genus is of great interest since it contains thale cress (Arabidopsis thaliana), one of the model organisms used for studying plant biology and the first plant to have its entire genome sequenced and is a popular tool for understanding the molecular biology of many plant traits, including flower development and light sensing. Changes in thale cress are easily observed, making it a very useful model.
Abscisic acid lays an important part in plant responses to environmental stress in response to decreased soil water potential.
Water Potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure, or matrix effects such as capillary action (which is caused by surface tension).
Clues to Possible Martian Life found in Chilean Desert. Samples contained unusual and highly specialized microbes that were
distributed in patches, which was linked to the scarce availability of water and nutrients.
Research provides insight on survivability of rare Wyoming plant - Desert Yellowhead (wiki)
Density Dependence processes occur when population growth rates are regulated by the density of a population. This article will focus on density-dependence in the context of macroparasite life cycles. Negative density-dependence, or density-dependent restriction, describes a situation in which population growth is curtailed by crowding, predators and competition. In cell biology, it describes the reduction in cell division. When a cell population reaches a certain density, the amount of required growth factors and nutrients available to each cell becomes insufficient to allow continued cell growth.
Interesting Facts about Water
There are about 5 sextillion atoms in a single drop of water. (1 with 21 zeros). One drop of water has the volume of about 0.05 mL.
There are more atoms in a glass of water than glasses of water in all the oceans on Earth.
50 Quintillion Atoms in a Grain of Sand. (50 with 18 zeros).
1 Nanometer is about the width of 2 Silicon Atoms? Nanometer is 1 Millionth of a Millimeter.
2 Trillion Hydrogen Atoms lined side by side to cross the head of a pin that is 1 mm in diameter?
(1 Million * 1 Million = 1 Trillion) - (mm is 0.0393701 inches or 25.4 mm in 1 inch).
Because the density of water is 1, the mass is 0.05 g.
The molar mass of water (H2O) is 18.0 grams/mol (1.008 + 1.008 + 16.0).
How many molecules in a drop of water?
This means there is one mole of water in 18.0 grams.
One mole is 6.02 × 1023 molecules. (10²³) - Avogadro Constant.
Then you can convert grams to number of atoms:
0.05 grams ÷ 18.0 grams × (6.02 × 1023 molecules) = 1.67 × 1021 molecule.
How Water conducts electricity, A watershed moment in understanding.
Dihydrogen Monoxide or Hydroxylic Acid?
Physics - Action Physics - Chemistry
What happens to Water in Space? (spheres)
Water in the Vacuum of Space
 Water has Memory II
- Prof. Bernd Kroeplin Water research Germany (youtube) -
The World in a Drop.
Water has Memory II
- Prof. Bernd Kroeplin Water research Germany (youtube) -
The World in a Drop."Our world view distinguishes between natural science and Humanities, between the world of the measurable and the world of unprovable notions. And these unprovable notions are also our feelings. And the world distinguishes between the spirit and the matter, and the shape, and the form and the content of the shape and the form. (from the book "The world in a drop" and the book "Water and its memory" by Prof. Dr. Bernd Kröplin. - Observation Effects - Sound Shapes.
"The mind permeates matter and that thoughts manifest themselves in material structuring's much more extensively than we now think possible."
Giving Thanks to clean water every time you drink it, will have positive effects, especially knowing that you are 70% water.
Water Memory (2014 Documentary about Nobel Prize laureate Luc Montagnier) (youtube)
The Mystery of Water - What we know is a drop (youtube)
Structured Water is a molecular arrangement of water molecules that exists when water is near hydrophilic (water loving) surfaces. The majority of the water in your body is structured water as your bodily tissues are hydrophilic, which is having a tendency to mix with, dissolve in, or be wetted by water. (Hexagonal water). Plants Create Structured Water.
H3O2 - Hydroxide, hydrate (Molecular Formula H3O2 - Average mass 35.023 Da - Monoisotopic mass 35.013851 Da - ChemSpider ID10632505). (Negative Charge on hydrophilic layer or material). Rain Clouds.
4 Phases of Water - Solid, Liquid, Gas and Gel or Jell-O phase which occurs next to water loving hydrophilic surfaces.
Two distinctly different liquid states of water. Using X-ray lasers, researchers have been able to follow the transformation between two distinct different liquid states of water. At around minus 63 Celsius, the two liquids exist at different pressure regimes with a density difference of 20 percent. By rapidly varying the pressure before the sample could freeze, it was possible to observe one liquid changing into the other in real time.
Quantum Coherent Water and Life - Water Basic Knowledge
Holy Water is water that has been blessed by a person.
Blessing is wishing for good things to happen to someone and for God or the Angles to look over them with gracious kindness as well as protect them from harm and evil.
Baptism the using water in a purification ritual and as a blessing that is also believed to cleanse someone from sin and awaken them to life in a special way. A small amount of water is either poured over a persons head, sometimes as they are standing or kneeling in water, or sometimes they are immersed or completely submerged under the water for a brief moment.
Coherent Domain. Anyone who has ever watched a school of fish in the ocean can understand what coherence is. Each fish is free to move independently, yet the group responds simultaneously—as a whole. It is as though the fish follow an unseen conductor, yet no conductor exists. In the world of physics, this kind of relationship is referred to as quantum coherence. At the smallest (quantum) level, there is unity and cooperation (coherence). This allows individual components to respond together as a larger unit.
Shapes from Sound - Water Therapy - Energy from Water
Magnetic and Electric Effects on Water
The Topography of Tears. Microscopic images of tears at different emotional states, like tears of joy and tears of grief. Even though Fisher uses scientific equipment and processes to make her images, the project isn't scientific, and it isn't meant to be. Rather than document her tears' biological properties, she instead reflects on "their existential and poetic nature." When making an image, Fisher does what so many photographers do: searches for meaning in what's in front of her.
Electromagnetic water cloak eliminates drag and wake. Researchers have developed a water cloaking concept based on electromagnetic forces that could eliminate an object's wake, greatly reducing its drag while simultaneously helping it avoid detection.
Rain has that certain smell, so why does rain have an odor? Petrichor is the earthy scent produced when rain falls on dry soil.
How Much Does One Ml Of Water Weigh At 4 Degrees Celsius?
Pure water has the highest density at the temperature of 3.98 degrees Celsius. The density is then 999.975 kg/m3 or 0.9999750 g/cm3 or 0.9999750 g/mL.
At 4°C pure water has a density (weight or mass) of about 1 g/cu.cm, 1 g/ml, 1 kg/litre, 1000 kg/cu.m, 1 tonne/cu.m. Water was used as the basis for establishing the metric unit of mass, however, so it is easier to remember that a cubic centimeter of it has a mass of 1 gm. Knowing that there are 1000 cubic centi-meters in a liter, you can also use 1 kilogram (1000 grams) per liter for water's mass density.
Properties of Water is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, nearly colorless with a hint of blue. The simplest hydrogen chalcogenide, it is by far the most studied chemical compound and is described as the "universal solvent" for its ability to dissolve many substances. This allows it to be the "solvent of life". It is the only common substance to exist as a solid, liquid, and gas in nature.
The magnetic fluctuations of the tides depend on the electrical conductivity of the water -- and the electrical conductivity of the water depends on its temperature. More than 90 percent of the excess heat in the Earth system goes into the ocean.
Water is the second most abundant substance in the universe. It dissolves more materials than any other solvent. It stores incredible amounts of energy. Life as we know it would not be possible without it. And although it covers more than 70% of the Earth’s surface, many parts of the world are in dire straits for lack of it. Water makes up 75% of our bodies. Every day we drink it, bathe in it, clean with it and use it to dispose of our wastes. Two atoms of hydrogen and one of oxygen. The hydrogen bonds that continually form and reform between its slightly negatively charged oxygen and slightly positively charged hydrogen components. Thanks to these bonds, water molecules attract one another far more strongly than those of almost any other substance. As it cools from its liquid to solid state, actually expands. Virtually every other substance becomes denser as it “freezes,” but thanks to this remarkable property, ice cubes float in our drinks. More importantly for living organisms, lakes and other bodies of water freeze from the top down. The average snow crystal contains about 10 quintillion (10 followed by 18 zeroes) water molecules, it is easy to see why the number of possible combinations is unimaginably large. Water has a cycle of evaporation, condensation, precipitation and runoff back to seas and lakes. The same is true among living organisms, where the hydrogen and oxygen constituents of water are continually combining and recombining through the processes of photosynthesis and respiration. Each time we break down a molecule of glucose, we produce six molecules of water, a reaction that takes place in the typical human body about six septillion (6 followed by 24 zeroes) times per day. Even so, we still don’t produce enough water to meet our own needs. 3% of the Earth’s water is fresh water, the other 97% being found in the oceans. And about 70% of this fresh water is found in glaciers and the ice caps of Antarctica. Earth holds enough water to make a sphere about 860 miles in diameter.
Tawainese local commercial farmers "cultivated the water", "stabilized the water", used "aged water" to mix with the water of the next growing cycle, transferred "good water" from a pond/tank to another recipient which the fish/shrimp were not growing well or were sick to "cure the water", etc. They used ancient Chinese principals and observations to help them to develop a "feeling for the water"! They even say that "you do not "grow the fish, you grow the water, fish is the result".
The resonant frequency of water is 42.7 khz.
Water Encapsulated in a Double Gelatinous Membrane.
Spherification is a culinary process that employs sodium alginate and either calcium chloride or calcium glucate lactate to shape a liquid into spheres, which visually and texturally resemble roe.
Sodium alginate (E-401) from the brown algae and Calcium Chloride (E-509) in a concrete proportions in order to generate a gelification on the exterior of the liquid. The final package is simple, cheap (2ct/unit), resistant, hygienic, biodegradable and even eatable.
Green Career
Colored Ferrofluid Displays (youtube) The Illumination' is a large ferrofluid display with a light in the base and reflective colored ferrofluid.
Water Philosophy
 Stand Like a Mountain, Flow Like Water - “Be like water making its way through
cracks. Do not be assertive, but adjust to the object, and you
shall find a way around or through it. If nothing within you
stays rigid, outward things will disclose themselves.
Empty your mind, be formless. Shapeless, like water. If you put
water into a cup, it becomes the cup. You put water into a
bottle and it becomes the bottle. You put it in a teapot, it
becomes the teapot. Now, water can flow or it can crash. Be like
water, my friend.” - (Bruce
Lee).
Stand Like a Mountain, Flow Like Water - “Be like water making its way through
cracks. Do not be assertive, but adjust to the object, and you
shall find a way around or through it. If nothing within you
stays rigid, outward things will disclose themselves.
Empty your mind, be formless. Shapeless, like water. If you put
water into a cup, it becomes the cup. You put water into a
bottle and it becomes the bottle. You put it in a teapot, it
becomes the teapot. Now, water can flow or it can crash. Be like
water, my friend.” - (Bruce
Lee).
"The green reed which bends in the wind is stronger than the mighty oak which breaks in a storm." Adapting.
Ride the Wave - Go with the Flow (don't swim against the tide or try to swim up stream unless you're a fish)
Let it Go is to allow someone or something to escape or go free. To relinquish one's grip on someone or something.
"The supreme goodness is like water. It benefits all things without contention. In dwelling, it stays grounded. In being, it flows to depths. In expression, it is honest. In confrontation, it stays gentle. In governance, it does not control. In action, it aligns to timing. It is content with its nature and therefore cannot be faulted." Tao Te Ching.
Tethys in mythology is the Greek Titan goddess of clean water. "Please help us again Tethys, we desperately need your support and your supreme wisdom to help guide us back to a more secure and healthy world."
"Everyone should experience water, and know what it feels like to swim like a fish. Either by snorkeling or by scuba diving. After all, we all were born in water, whether in the womb, or in the sea. Water is Life."