BK101
Knowledge Base
Physics
Physics is the science of matter and energy and their interactions and motion through space and time. The science of change, including physical laws, physical properties and phenomena. Observations.
Laws - Chemistry - Scopes - Atoms - Quantum Physics - Particles - Waves
Physicist is a scientist who has specialized knowledge in the field of physics, which encompasses the interactions of matter and energy at all length and time scales in the physical universe. Physicists generally are interested in the root or ultimate causes of phenomena, and usually frame their understanding in mathematical terms. Physicists work across a wide range of research fields, spanning all length scales: from sub-atomic and particle physics, to molecular length scales of chemical and biological interest, to cosmological length scales encompassing the Universe as a whole. The field generally includes two types of physicists: experimental physicists who specialize in the observation of physical phenomena and the analysis of experiments, and theoretical physicists who specialize in mathematical modeling of physical systems to rationalize, explain and predict natural phenomena. Physicists can apply their knowledge towards solving practical problems or developing new technologies. (also known as applied physics or engineering physics). "Physicists do a lot of research to learn more and understand more about our world, and in the process they make many new discoveries. Then Engineers will build and develop new tools and products using this newly discovered knowledge that physicists provided from years of research".
Classical Physics refers to theories of physics that predate modern, more complete, or more widely applicable theories. If a currently accepted theory is considered to be "modern," and its introduction represented a major paradigm shift, then the previous theories, or new theories based on the older paradigm, will often be referred to as belonging to the realm of "classical" physics.
Physics has learned a lot about matter and energy and how it works and behaves. But we are still only scratching the surface of what is known. We live on speck of dust in a universe that is so large that we can't even see where the universe ends, and we can't even see how small things are or how small things can get, so we still have a lot more to learn. So shut up and calculate or shut up and contemplate.
Physical Law is a theoretical statement "inferred from particular facts, applicable to a defined group or class of phenomena, and expressible by the statement that a particular phenomenon always occurs if certain conditions be present." Physical laws are typically conclusions based on repeated scientific experiments and observations over many years and which have become accepted universally within the scientific community. The production of a summary description of our environment in the form of such laws is a fundamental aim of science. These terms are not used the same way by all authors. Fundamental Physics Formulas (wiki) - Forces of Nature (constants).
Phenomena is any thing which manifests itself. Phenomena are often, but not always, understood as "things that appear" or "experiences" for a sentient being, or in principle may be so. To show, shine, appear, to be manifest or manifest itself, plural phenomena).
Particle Physics is the branch of physics that studies the nature of the particles that constitute matter (particles with mass) and radiation (massless particles). Waves - Particle Accelerator.
Atomic Physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. It is primarily concerned with the arrangement of electrons around the nucleus and the processes by which these arrangements change. This comprises ions, neutral atoms and, unless otherwise stated, it can be assumed that the term atom includes ions.
Atomic fingerprint is a term for the unique line spectrum that is characteristic of a given element and can be used for identification. Balmer Formula is a mathematical equation for calculating the emitted wavelength of a light from an excited hydrogen atom when an electron drops to the second energy level. When an electron “drops” to a lower energy level, that electron ends up with less energy than it had originally. That “lost” energy doesn’t just disappear, it’s not destroyed, it has to go somewhere. What happens is that that energy is transformed into another type of energy. In the case of these atoms, it’s transformed into electromagnetic energy; sometimes visible light, sometimes X-rays, sometimes other.
Nuclear Physics is the field of physics that studies atomic nuclei and their constituents and interactions. The most commonly known application of nuclear physics is nuclear power generation, but the research has led to applications in many fields, including nuclear medicine and magnetic resonance imaging, nuclear weapons, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology.
E=MC2 - Action Physics is the study of Motion.
Digital Physics is a collection of theoretical perspectives based on the premise that the universe is, at heart, describable by information. Therefore, according to this theory, the universe can be conceived of as either the output of a deterministic or probabilistic computer program, a vast, digital computation device, or mathematically isomorphic to such a device.
Digital Philosophy is a modern re-interpretation that all information must have a digital means of its representation. An informational process transforms the digital representation of the state of the system into its future state. The world can be resolved into digital bits, with each bit made of smaller bits. These bits form a fractal pattern in fact-space. The pattern behaves like a cellular automaton. The pattern is inconceivably large in size and dimensions. Although the world started simply, its computation is irreducibly complex.
Theoretical Physics employs mathematical models and abstractions of physical objects and systems to rationalize, explain and predict natural phenomena. This is in contrast to experimental physics, which uses experimental tools to probe these phenomena.
Experimental Physics is the category of disciplines and sub-disciplines in the field of physics that are concerned with the observation of physical phenomena and experiments. Methods vary from discipline to discipline, from simple experiments and observations, such as the Cavendish experiment, to more complicated ones, such as the Large Hadron Collider.
Mathematical Physics refers to development of mathematical methods for application to problems in physics.
Science Research
Plasma Physics: Plasma can be created by heating a gas or subjecting it to a strong electromagnetic field, applied with a laser or microwave generator at temperatures above 5000 °C. This decreases or increases the number of electrons in the atoms or molecules, creating positive or negative charged particles called ions, and is accompanied by the dissociation of molecular bonds, if present. Plasma is the most abundant form of ordinary matter in the universe.
Fusion (cold fusion)
Astrophysics is the branch of astronomy that employs the principles of physics and chemistry "to ascertain the nature of the heavenly bodies, rather than their positions or motions in space.
Metaphysics (philosophy)
Biophysics is an interdisciplinary science that applies the approaches and methods of physics to study biological systems. Biophysics covers all scales of biological organization, from molecular to organismic and populations. Biophysical research shares significant overlap with biochemistry, physical chemistry, nanotechnology, bioengineering, computational biology, biomechanics and systems biology. Electromagnetic Radiation - Mitochondria.
If one wants to summarize our knowledge of physics in the briefest possible terms, there are three really fundamental observations: (i) Space-time is a pseudo-Riemannian manifold M, endowed with a metric tensor and governed by geometrical laws. (ii) Over M is a vector bundle X with a nonabelian gauge group G. (iii) Fermions are sections of (S~+⊗VR)⊕(S~−⊗VR~). R and R~ are not isomorphic; their failure to be isomorphic explains why the light fermions are light and presumably has its origins in a representation difference Δ in some underlying theory. All of this must be supplemented with the understanding that the geometrical laws obeyed by the metric tensor, the gauge fields, and the fermions are to be interpreted in quantum mechanical terms.
Einstein Field Equations relate the geometry of space-time with the distribution of matter within it
Dirac Equation is a relativistic wave equation describes all spin-1/2 massive particles such as electrons and quarks for which parity is a symmetry. It is consistent with both the principles of quantum mechanics and the theory of special relativity, and was the first theory to account fully for special relativity in the context of quantum mechanics. It was validated by accounting for the fine details of the hydrogen spectrum in a completely rigorous way.
Mills Theory is a gauge theory based on a special unitary group SU(N), or more generally any compact, reductive Lie algebra. Yang–Mills theory seeks to describe the behavior of elementary particles using these non-abelian Lie groups and is at the core of the unification of the electromagnetic force and weak forces (i.e. U(1) × SU(2)) as well as quantum chromodynamics, the theory of the strong force (based on SU(3)). Thus it forms the basis of our understanding of the Standard Model of particle physics.
Forces of Nature - Laws
1: Weak Force is responsible for radioactive decay, which plays an essential role in nuclear fission. Weak Force is also known as Weak Interaction or Weak Nuclear Force. Electroweak Interaction is the unified description of two of the four known fundamental interactions of nature, which are electromagnetism and the weak interaction. Physical Law - Scientific Law.
2: Strong Force is the mechanism responsible for holding atoms together. Strong force is also called strong interaction, the strong force, nuclear strong force or strong nuclear force. At the range of a femtometer, it is the strongest force, being approximately 137 times stronger than electromagnetism, a million times stronger than weak interaction and 1038 times stronger than gravitation. The strong nuclear force ensures the stability of ordinary matter, confining quarks into hadron particles, such as the proton and neutron, and the further binding of neutrons and protons into atomic nuclei. Most of the mass-energy of a common proton or neutron is in the form of the strong force field energy; the individual quarks provide only about 1% of the mass-energy of a proton.
3: Electromagnetic Force is a type of physical interaction that occurs between electrically charged particles. Waves.
4: Gravity is the force of attraction between all masses in the universe; especially the attraction of the earth's mass for bodies near its surface. All things with energy are brought toward or gravitate toward one another, including stars, planets, galaxies and even light and sub-atomic particles. Action Physics.
Fifth Force? - A Description is not an Explanation - Core Theory of Physics (image of equation)
Theory of Everything is a theoretical framework of physics that links together all the physical aspects of the universe into a single coherent explanation.
Standard Model is a theory concerning the electromagnetic, weak, and strong nuclear interactions, as well as classifying all the subatomic particles known.
Penguin Diagram are a class of Feynman diagrams which are important for understanding CP violating processes in the standard model. They refer to one-loop processes in which a quark temporarily changes flavor (via a W or Z loop), and the flavor-changed quark engages in some tree interaction, typically a strong one. For the interactions where some quark flavors (e.g. very heavy ones) have much higher interaction amplitudes than others, such as CP-violating or Higgs interactions, these penguin processes may have amplitudes comparable to or even greater than those of the direct tree processes. A similar diagram can be drawn for leptonic decays.
Fundamental Interaction are the interactions that do not appear to be reducible to more basic interactions. There are four fundamental interactions known to exist: the gravitational and electromagnetic interactions, which produce significant long-range forces whose effects can be seen directly in everyday life, and the strong and weak interactions, which produce forces at minuscule, subatomic distances and govern nuclear interactions. Some scientists hypothesize that a fifth force might exist, but these hypotheses remain speculative. (also known as fundamental forces).
Uniformitarianism was an assumption that the same natural laws and processes that operate in the universe now have always operated in the universe in the past and apply everywhere in the universe. Physical Constant (consistent)
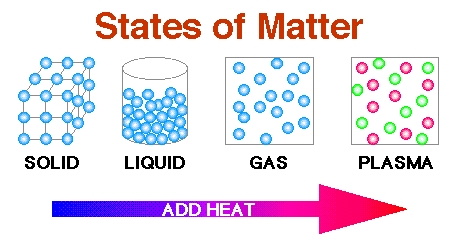
States of Matter
1: Gas may be made up of individual atoms like with a noble gas, or elemental molecules made from one type of atom like with oxygen, or compound molecules made from a variety of atoms like carbon dioxide. A gas mixture would contain a variety of pure gases much like the air. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer. The interaction of gas particles in the presence of electric and gravitational fields are considered negligible as indicated by the constant velocity vectors in the image. One type of commonly known gas is steam. Evaporation.
2: Solid is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a gas does. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice) or irregularly (an amorphous solid such as common window glass). Rocks - Minerals - Particles.
3: Liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma), and is the only state with a definite volume but no fixed shape. A liquid is made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds. Water is, by far, the most common liquid on Earth. Like a gas, a liquid is able to flow and take the shape of a container. Most liquids resist compression, although others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension, leading to wetting phenomena.
4: Plasma is a gas that becomes heated until the atoms lose all their electrons, leaving a highly electrified collection of nuclei and free electrons. A plasma has properties unlike those of the other three states. A plasma can be created by heating a gas or subjecting it to a strong electromagnetic field, applied with a Laser or microwave generator. This decreases or increases the number of electrons, creating positive or negative charged particles called ions, and is accompanied by the dissociation of molecular bonds, if present. Plasma Oscillation are rapid oscillations of the electron density in conducting media such as plasmas or metals. The oscillations can be described as an instability in the dielectric function of a free electron gas. The frequency only depends weakly on the wavelength of the oscillation. The quasiparticle resulting from the quantization of these oscillations is the plasmon. Plasma is the most common form of matter as most of the universe is composed of stars. Nonthermal Plasma is a plasma which is not in thermodynamic equilibrium, because the electron temperature is much hotter than the temperature of heavy species (ions and neutrals). As only electrons are thermalized, their Maxwell-Boltzmann velocity distribution is very different than the ion velocity distribution. When one of the velocities of a species does not follow a Maxwell-Boltzmann distribution, the plasma is said to be non-Maxwellian. A kind of common nonthermal plasma is the mercury-vapor gas within a fluorescent lamp, where the "electron gas" reaches a temperature of 20,000 K (19,700 °C; 35,500 °F) while the rest of the gas, ions and neutral atoms, stays barely above room temperature, so the bulb can even be touched with hands while operating. Where does laser energy go after being fired into plasma? - Plasma Physics - Plasma Universe.
 State of
Matter is one of the distinct forms in which matter can exist.
Four
States of Matter are Observable in Everyday Life: solid, liquid, gas, and
plasma. Many other states are known to exist, such as glass or liquid
crystal, and some only exist under extreme conditions, such as
Bose–Einstein condensates, neutron-degenerate matter, and quark-gluon
plasma, which only occur, respectively, in situations of extreme cold,
extreme density, and extremely high-energy. Some other states are believed
to be possible but remain theoretical for now. For a complete list of all
exotic states of matter,
List of States of Matter (wiki).
State of
Matter is one of the distinct forms in which matter can exist.
Four
States of Matter are Observable in Everyday Life: solid, liquid, gas, and
plasma. Many other states are known to exist, such as glass or liquid
crystal, and some only exist under extreme conditions, such as
Bose–Einstein condensates, neutron-degenerate matter, and quark-gluon
plasma, which only occur, respectively, in situations of extreme cold,
extreme density, and extremely high-energy. Some other states are believed
to be possible but remain theoretical for now. For a complete list of all
exotic states of matter,
List of States of Matter (wiki).
Surface Science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics, which is the study of physical interactions that occur at interfaces. It overlaps with surface chemistry. Some of the things investigated by surface physics include friction, surface states, surface diffusion, surface reconstruction, surface phonons and plasmons, epitaxy and surface enhanced Raman scattering, the emission and tunneling of electrons, spintronics, and the self-assembly of nanostructures on surfaces. In a confined liquid, defined by geometric constraints on a nanoscopic scale, most molecules sense some surface effects, which can result in physical properties grossly deviating from those of the bulk liquid. Surface Chemistry - Cell Surface - Surface Engineering.
Absorption in chemistry is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter some bulk phase – liquid or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface (as in the case for adsorption). A more general term is sorption, which covers absorption, adsorption, and ion exchange. Absorption is a condition in which something takes in another substance.
Physicists have identified a new state of matter whose structural order operates by rules more aligned with quantum mechanics than standard thermodynamic theory. In a classical material called artificial spin ice, which in certain phases appears disordered, the material is actually ordered, but in a "topological" form.
Spin Ice is a magnetic substance that does not have a single minimal-energy state. It has magnetic moments (i.e. "spin") as elementary degrees of freedom which are subject to frustrated interactions. By their nature, these interactions prevent the moments from exhibiting a periodic pattern in their orientation down to a temperature much below the energy scale set by the said interactions. Spin ices show low-temperature properties, residual entropy in particular, closely related to those of common crystalline water ice. (Shakti spin ice).
New State of Physical Matter in which atoms can exist as both Solid and Liquid simultaneously. Applying high pressures and temperatures to potassium -- a simple metal -- creates a state in which most of the element's atoms form a solid lattice structure, the findings show. However, the structure also contains a second set of potassium atoms that are in a fluid arrangement. Under the right conditions, over half a dozen elements -- including sodium and bismuth -- are thought to be capable of existing in the newly discovered state, researchers say.
Matter
Matter is something which has mass and occupies space. Matter includes atoms and molecules and anything made up of these, but not other energy phenomena or waves such as light or sound. Elements.
Substance is the real physical matter of which a person or thing consists. The choicest or most essential or most vital part of some idea or experience. A particular kind or species of matter with uniform properties. The property of holding together and retaining its shape. Mineral.
Material is the tangible substance that goes into the makeup of a physical object. Something derived from or composed of matter and having physical form or substance. Something physical as distinct from intellectual or psychological well-being. Something that is real rather than spiritual or abstract, though information, data or ideas and observations can be used or reworked into a finished form. Material Science - Immaterial.
Physical is something having substance or material existence and perceptible to the senses. Something characterized by energetic bodily activity, matter and energy.
State of Matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma.
Pyramid of Complexity - Dark Matter
Phase in matter is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air over the water is a third phase. The glass of the jar is another separate phase.
Existence of New Form of Electronic Matter Quadrupole Topological Insulators
Baryonic Matter nearly all matter that may be encountered or experienced in everyday life is baryonic matter, which includes atoms of any sort, and provides those with the property of mass. Non-baryonic matter, as implied by the name, is any sort of matter that is not composed primarily of baryons. This might include neutrinos and free electrons, dark matter, such as supersymmetric particles, axions, and black holes. Baryon is a composite subatomic Particle made up of three Quarks.
Baryon Asymmetry problem in physics refers to the imbalance in baryonic matter (the type of matter experienced in everyday life) and antibaryonic matter in the observable universe. Neither the standard model of particle physics, nor the theory of general relativity provides an obvious explanation for why this should be so, and it is a natural assumption that the universe be neutral with all conserved charges. The Big Bang should have produced equal amounts of matter and antimatter. Since this does not seem to have been the case, it is likely some physical laws must have acted differently or did not exist for matter and antimatter. Several competing hypotheses exist to explain the imbalance of matter and antimatter that resulted in baryogenesis. However, there is as of yet no consensus theory to explain the phenomenon. As remarked in a 2012 research paper, "The origin of matter remains one of the great mysteries in physics.
Condensed Matter Physics is a branch of physics that deals with the physical properties of condensed phases of matter, where particles adhere to each other. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, they include the laws of quantum mechanics, electromagnetism and statistical mechanics.
Programmable Matter is matter which has the ability to change its physical properties (shape, density, moduli, conductivity, optical properties, etc.) in a programmable fashion, based upon user input or autonomous sensing. Programmable matter is thus linked to the concept of a material which inherently has the ability to perform information processing.
Does Matter Die? Stars create new elements in their cores by squeezing elements together in a process called nuclear fusion. But if mass can neither be created nor destroyed, then how does the Sun create different Atoms?
Dark Matter - Time Crystals
Antimatter is a material composed of anti-particles, which have the same mass as particles of ordinary matter, but opposite charges, lepton numbers, and baryon numbers.
In 1928, a physicist named Paul Dirac found something strange in his equations. And he predicted, based purely on mathematical insight, that there ought to be a second kind of matter, the opposite to normal matter, that literally annihilates when it comes in contact. Antimatter.
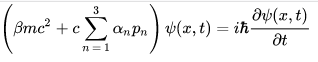
Annihilation is the process that occurs when a subatomic particle collides with its respective Antiparticle to produce other particles, such as an electron colliding with a positron to produce two photons. The total energy and momentum of the initial pair are conserved in the process and distributed among a set of other particles in the final state. Antiparticles have exactly opposite additive quantum numbers from particles, so the sums of all quantum numbers of such an original pair are zero. Hence, any set of particles may be produced whose total quantum numbers are also zero as long as conservation of energy and conservation
of momentum are obeyed. During a low-energy annihilation, photon production is favored, since these particles have no mass. However, high-energy particle colliders produce annihilations where a wide variety of exotic heavy particles are created. The word annihilation takes use informally for the interaction of two particles that are not mutual antiparticles - not charge conjugate. Some quantum numbers may then not sum to zero in the initial state, but conserve with the same totals in the final state. An example is the "annihilation" of a high-energy electron antineutrino with an electron to produce a W-. If the annihilating particles are composite, such as mesons or baryons, then several different particles are typically produced in the final state.
Hollow Atoms are short-lived multiply excited neutral atoms which carry a large part of their Z electrons (Z ... projectile nuclear charge) in high-n levels while inner shells remain (transiently) empty. This population inversion arises for typically 100 femtoseconds during the interaction of a slow highly charged ion (HCI) with a solid surface. Despite this limited lifetime, the formation and decay of a hollow atom can be conveniently studied from ejected electrons and soft X-rays, and the trajectories, energy loss and final charge state distribution of surface-scattered projectiles. For impact on insulator surfaces the potential energy contained by hollow atom may also cause the release of target atoms and -ions via potential sputtering and the formation of nanostructures on a surface.
Possible explanation for the dominance of matter over antimatter in the Universe. Neutrinos and antineutrinos, sometimes called ghost particles because difficult to detect, can transform from one type to another. The international T2K Collaboration announces a first indication that the dominance of matter over antimatter may originate from the fact that Neutrinos and antineutrinos behave differently during those oscillations. Neutrinos are elementary particles which travel through matter almost without interaction. They appear in three different types: electron- muon- and tau-neutrinos and their respective antiparticle (antineutrinos).
Exotic Matter has several proposed types: Hypothetical particles and states of matter that have "exotic" physical properties that would violate known laws of physics, such as a particle having a negative mass. Hypothetical particles and states of matter that have not yet been encountered, but whose properties would be within the realm of mainstream physics if found to exist. Several particles whose existence has been experimentally confirmed that are conjectured to be exotic hadrons and within the Standard Model. States of matter that are not commonly encountered, such as Bose–Einstein condensates, fermionic condensates, quantum spin liquid, string-net liquid, supercritical fluid, color-glass condensate, quark–gluon plasma, Rydberg matter, Rydberg polaron and photonic matter but whose properties are entirely within the realm of mainstream physics. Forms of matter that are poorly understood, such as dark matter and mirror matter. Ordinary matter placed under high pressure, which may result in dramatic changes in its physical or chemical properties. Degenerate matter. Exotic atoms.
Mass
Mass is both a property of a physical body and a measure of its resistance to acceleration (a change in its state of motion) when a net force is applied. An object's mass also determines the strength of its gravitational attraction to other bodies. The basic SI unit of mass is the kilogram (kg). In physics, mass is not the same as weight, even though mass is often determined by measuring the object's weight using a spring scale, rather than balance scale comparing it directly with known masses. An object on the Moon would weigh less than it does on Earth because of the lower gravity, but it would still have the same mass. This is because weight is a force, while mass is the property that (along with gravity) determines the strength of this force. Mass is the property of a body that causes it to have weight in a gravitational field. Join together into a mass or collect or form a mass. Mass is a property of a physical body. It is the measure of an object's resistance to Acceleration (a change in its state of motion) when a net force is applied. It also determines the strength of its mutual gravitational attraction to other bodies. The basic SI unit of mass is the kilogram (kg). Mass is not the same as weight, even though we often calculate an object's mass by measuring its weight with a spring scale, rather than comparing it directly with known masses. An object on the Moon would weigh less than it does on Earth because of the lower gravity, but it would still have the same mass. This is because weight is a force, while mass is the property that (along with gravity) determines the strength of this Force.
Physical Object is a collection of matter within a defined contiguous boundary in three-dimensional space. The boundary must be defined and identified by the properties of the material. The boundary may change over time. The boundary is usually the visible or tangible surface of the object. The matter in the object is constrained (to a greater or lesser degree) to move as one object. The boundary may move in space relative to other objects that it is not attached to (through translation and rotation). An object's boundary may also deform and change over time in other ways.
Physical Property is any property that is measurable, whose value describes a state of a physical system. The changes in the physical properties of a system can be used to describe its transformations or evolutions between its momentary states. Physical properties are often referred to as observables. They are not modal properties. Quantifiable physical property is called physical quantity. Environment.
Mass in Special Relativity. The word mass has two meanings in special relativity: rest mass or invariant mass is an invariant quantity which is the same for all observers in all reference frames, while relativistic mass is dependent on the velocity of the observer. According to the concept of mass–energy equivalence, the rest mass and relativistic mass are equivalent to the rest energy and total energy of the body, respectively. The term relativistic mass tends not to be used in particle and nuclear physics and is often avoided by writers on special relativity, in favor of using the body's total energy. In contrast, rest mass is usually preferred over rest energy. The measurable inertia and gravitational attraction of a body in a given frame of reference is determined by its relativistic mass, not merely its rest mass. For example, light has zero rest mass but contributes to the inertia (and weight in a gravitational field) of any system containing it. For a discussion of mass in general relativity, see mass in general relativity. For a general discussion including mass in Newtonian mechanics, see the article on mass.
E = mc²
Energy Equals Mass Times The Speed Of Light Squared. The equation says that energy and mass or matter are interchangeable; they are different forms of the same thing. Under the right conditions, energy can become mass, and vice versa. Theory of Relativity.
Mass Energy Equivalence states that anything having mass has an equivalent amount of energy and vice versa, with these fundamental quantities directly relating to one another by Albert Einstein's famous formula: E=mc2 - Simple Version. This formula states that the equivalent energy (E) can be calculated as the mass (m) multiplied by the speed of light (c = about 3×108 m/s) squared. Similarly, anything having energy exhibits a corresponding mass m given by its energy E divided by the speed of light squared c². Because the speed of light is a very large number in everyday units, the formula implies that even an everyday object at rest with a modest amount of mass has a very large amount of energy intrinsically. Chemical, nuclear, and other energy transformations may cause a system to lose some of its energy content (and thus some corresponding mass), releasing it as light (radiant) or thermal energy for example.
Mass and Energy are manifestations of the same thing? The mass of a body is a measure of its energy content. Mass becomes simply a physical manifestation of that energy, rather than the other way around. As we work our way inward—matter into atoms, atoms into sub-atomic particles, sub-atomic particles into quantum fields and forces—we lost sight of matter completely. Matter loses its tangibility. It lost its primacy as mass became a secondary quality, the result of interactions between intangible quantum fields. What we recognize as mass is a behavior of these quantum fields; it is not a property that belongs or is necessarily intrinsic to them. Mass overwhelmingly arises from the protons and neutrons it contains, the answer is now clear and decisive. The inertia of that body, with 95 percent accuracy, is its energy content.
Mass is only one form of energy among many, such as electrical, thermal, or chemical energy, and therefore energy can be transformed from any of these forms into mass, and vice versa. Converting mass into energy is the most energy-efficient process in the Universe. 100% is the greatest energy gain you could ever hope for out of a reaction. Mass can be converted into energy and back again, and underlies everything from nuclear power to particle accelerators to atoms to the Solar System. Mass is not conserved. If you take a block of iron and chop it up into a bunch of iron atoms, you fully expect that the whole equals the sum of its parts. That's an assumption that's clearly true, but only if mass is conserved. In the real world, though, according to Einstein, mass is not conserved at all. If you were to take an iron atom, containing 26 protons, 30 neutrons, and 26 electrons, and were to place it on a scale, you'd find some disturbing facts. An iron atom with all of its electrons weighs slightly less than an iron nucleus and its electrons do separately, An iron nucleus weighs significantly less than 26 protons and 30 neutrons do separately. And if you try and fuse an iron nucleus into a heavier one, it will require you to input more energy than you get out. mass is just another form of energy. When you create something that's more energetically stable than the raw ingredients that it's made from, the process of creation must release enough energy to conserve the total amount of energy in the system. When you bind an electron to an atom or molecule, or allow those electrons to transition to the lowest-energy state, those binding transitions must give off energy, and that energy must come from somewhere: the mass of the combined ingredients. This is even more severe for nuclear transitions than it is for atomic ones, with the former class typically being about 1000 times more energetic than the latter class. Energy is conserved, but only if you account for changing masses. When you have any attractive force that binds two objects together — whether that's the electric force holding an electron in orbit around a nucleus, the nuclear force holding protons and neutrons together, or the gravitational force holding a planet to a star — the whole is less massive than the individual parts. And the more tightly you bind these objects together, the more energy the binding process emits, and the lower the rest mass of the end product. For every 1 kilogram of mass that you convert, you get a whopping 9 × 1016 joules of energy out: the equivalent of 21 Megatons of TNT. Whenever we experience a radioactive decay, a fission or fusion reaction, or an annihilation event between matter and antimatter, the mass of the reactants is larger than the mass of the products; the difference is how much energy is released. In all cases, the energy that comes out — in all its combined forms — is exactly equal to the energy equivalent of the mass loss between products and reactants. The ultimate example is the case of matter-antimatter annihilation, where a particle and its antiparticle meet and produce two photons of the exact rest energy of the two particles. Take an electron and a positron and let them annihilate, and you'll always get two photons of exactly 511 keV of energy out. It's no coincidence that the rest mass of electrons and positrons are each 511 keV/c2: the same value, just accounting for the conversion of mass into energy by a factor of c2. Einstein's most famous equation teaches us that any particle-antiparticle annihilation has the potential to be the ultimate energy source: a method to convert the entirety of the mass of your fuel into pure, useful energy.
Explosion is a rapid increase in volume and release of energy in an extreme manner, usually with the generation of high temperatures and the release of gases. Supersonic explosions created by high explosives are known as detonations and travel via supersonic shock waves. Subsonic explosions are created by low explosives through a slower burning process known as deflagration.
Combustion - Rockets - Chemical Reactions
Coulomb Explosion are a mechanism for transforming energy in intense electromagnetic fields into atomic motion and are thus useful for controlled destruction of relatively robust molecules. The explosions are a prominent technique in laser-based machining, and appear naturally in certain high-energy reactions.
Implosion as a mechanical process is when objects are destroyed by collapsing (or being squeezed in) on themselves. The opposite of explosion, implosion concentrates matter and energy. True implosion usually involves a difference between internal (lower) and external (higher) pressure, or inward and outward forces, that is so large that the structure collapses inward into itself, or into the space it occupied if it is not a completely solid object. Examples of implosion include a submarine being crushed from the outside by the hydrostatic pressure of the surrounding water, and the collapse of a massive star under its own gravitational pressure. An implosion can fling material outward (for example due to the force of inward falling material rebounding, or peripheral material being ejected as the inner parts collapse), but this is not an essential component of an implosion and not all kinds of implosion will do so. If the object was previously solid, then implosion usually requires it to take on a more dense form - in effect to be more concentrated, compressed, denser, or converted into a new material that is denser than the original. Cavitation - Contraction - Impeller.
Exothermic Process describes a process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen). Its etymology stems from the Greek prefix έξω (exō, which means "outwards") and the Greek word θερμικός (thermikόs, which means "thermal"). The term exothermic was first coined by Marcellin Berthelot. The opposite of an exothermic process is an endothermic process, one that absorbs energy in the form of heat. Combustion.
Mass versus Weight, the mass of an object is often referred to as its weight, though these are in fact different concepts and quantities. In scientific contexts, mass refers loosely to the amount of "matter" in an object (though "matter" may be difficult to define), whereas weight refers to the force exerted on an object by gravity. In other words, an object with a mass of 1.0 kilogram will weigh approximately 9.81 newtons on the surface of the Earth (its mass multiplied by the gravitational field strength). (The newton is a unit of force, while the kilogram is a unit of mass.)
Energy Types - Light
Invariant Mass is a characteristic of the total energy and momentum of an object or a system of objects that is the same in all frames of reference related by Lorentz transformations. If a center of momentum frame exists for the system, then the invariant mass of a system is simply the total energy divided by the speed of light squared. In other reference frames, the energy of the system increases, but system momentum is subtracted from this, so that the invariant mass remains unchanged.
Negative Mass is a hypothetical concept of matter whose mass is of opposite sign to the mass of normal matter, e.g. −2 kg. Such matter would violate one or more energy conditions and show some strange properties, stemming from the ambiguity as to whether attraction should refer to force or the oppositely oriented acceleration for negative mass. It is used in certain speculative theories, such as on the construction of wormholes. The closest known real representative of such exotic matter is a region of pseudo-negative pressure density produced by the Casimir effect, which are physical forces arising from a quantized field, which is the process of transition from a classical understanding of physical phenomena to a newer understanding known as quantum mechanics. It is a procedure for constructing a quantum field theory starting from a classical field theory. Dark Energy.
Inertial mass is a mass parameter giving the inertial resistance to acceleration of the body when responding to all types of force. Gravitational mass is determined by the strength of the gravitational force experienced by the body when in the gravitational field g.
Casimir Effect typical example is of the two uncharged conductive plates in a vacuum, placed a few nanometers apart. In a classical description, the lack of an external field means that there is no field between the plates, and no force would be measured between them. When this field is instead studied using the quantum electrodynamic vacuum, it is seen that the plates do affect the virtual photons which constitute the field, and generate a net force – either an attraction or a repulsion depending on the specific arrangement of the two plates. Although the Casimir effect can be expressed in terms of virtual particles interacting with the objects, it is best described and more easily calculated in terms of the Zero Point Energy of a quantized field in the intervening space between the objects. This force has been measured and is a striking example of an effect captured formally by second quantization. The treatment of boundary conditions in these calculations has led to some controversy. In fact, "Casimir's original goal was to compute the van der Waals force between polarizable molecules" of the conductive plates. Thus it can be interpreted without any reference to the zero-point energy (vacuum energy) of quantum fields. Because the strength of the force falls off rapidly with distance, it is measurable only when the distance between the objects is extremely small. On a submicron scale, this force becomes so strong that it becomes the dominant force between uncharged conductors. In fact, at separations of 10 nm – about 100 times the typical size of an atom – the Casimir effect produces the equivalent of about 1 atmosphere of pressure (the precise value depending on surface geometry and other factors).
Zero Point Energy - Quantized Energy - Anti-Gravity - Magnets
Biefeld–Brown Effect is an electrical effect that produces an ionic wind that transfers its momentum to surrounding neutral particles.
Pauli Exclusion Principle is the quantum mechanical principle which states that two or more identical fermions (particles with half-integer spin) cannot occupy the same quantum state within a quantum system simultaneously. In the case of electrons in atoms, it can be stated as follows: it is impossible for two electrons of a poly-electron atom to have the same values of the four quantum numbers.
Cavitation is the formation of vapour cavities in a liquid, small liquid-free zones ("bubbles" or "voids"), that are the consequence of forces acting upon the liquid. It usually occurs when a liquid is subjected to rapid changes of pressure that cause the formation of cavities in the liquid where the pressure is relatively low. When subjected to higher pressure, the voids implode and can generate an intense shock wave.
Elements - Minerals - Mind over Matter
‘Negative mass’ created at Washington State University
Negative Energy is a concept used in physics to explain the nature of certain fields, including the gravitational field and a number of quantum field effects. In more speculative theories, negative energy is involved in wormholes which allow time travel and warp drives for faster-than-light space travel.
Atoms - Matter - Tiny Particles
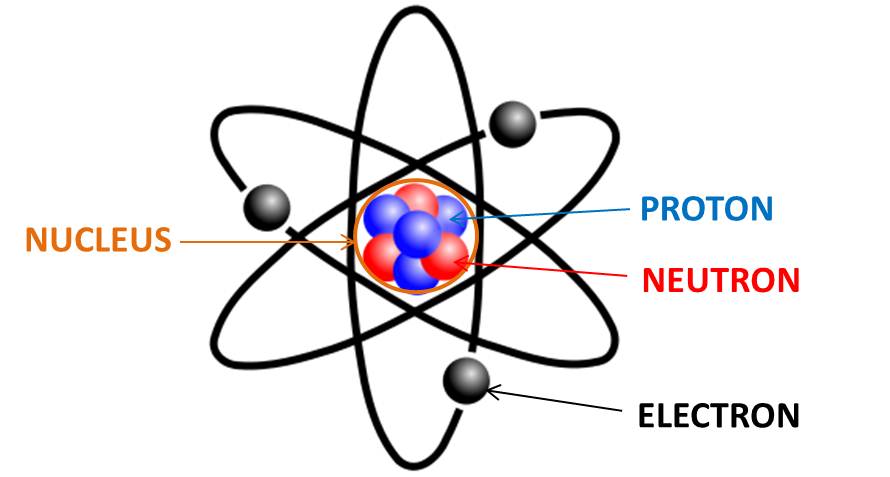 An
Atom is the smallest constituent
unit of ordinary
matter that has the properties of a
chemical element. Every solid,
liquid, gas, and plasma is composed of neutral or ionized atoms.
Atoms with electrons are 99.9% empty space.
An
Atom is the smallest constituent
unit of ordinary
matter that has the properties of a
chemical element. Every solid,
liquid, gas, and plasma is composed of neutral or ionized atoms.
Atoms with electrons are 99.9% empty space.
Atomic Number - Atoms (youtube) - Atoms (youtube)
Atoms are very small around ten-billionth of a meter, or in the short scale, roughly 1 angstrom across = one ten millionth of a mm. (1,000 mm in 1 meter). One nanometer is the size of two atoms and a nanometer is one millionth of a millimeter or the size of a small grain of sand. A hydrogen atom is about 0.1 nanometers. Atom is about 0.0000001 of a millimeter in diameter, or 0.1 nanometers. However, atoms do not have well-defined boundaries, and there are different ways to define their size that give different but close values. The nucleus accounts for 99.9% of an atom's mass. So what would be the size difference of an atom if you only measure the size of the protons and neutrons and did not measure the space or the electrons? How many atoms in single drop of water? If a single atom were the size of a football stadium the nucleus of the atom would be the size of your eyeball, and the electrons circling the stadium would be invisible to you. The atom nucleus is 10,000 times smaller than the radius of the circling electrons. If an atoms outer electron layer were the size of a basketball, the nucleus of the atom would be so small that you could not see it with your own eyes. An atom is 99.9% empty space. The world gets really weird at that scale. If the atom were the size of a human eye ball, and you had a proton spinning in the palm of your hand, that means that you are just a little bit bigger than an atom, which means that the next proton would be so far away that you could not see it. Life at that scale would look like empty space to a human.
Size Scales (nano) - Ions - Relativity - Weighing Atoms with Electrons
All Atoms have at least one proton in their core, and the number of protons determines which kind of element an atom is. All atoms have electrons, negatively charged particles that move around in the space surrounding the positively-charged nuclear core. Hydrogen has one proton, one electron and no neutrons. An atom has a positively charged core. The core is surrounded by negatively charged electrons. The electrons spin around the core of the atom. This turns the atom into a tiny magnet. Each atom in an object creates a small magnetic force. In most materials, the atoms align in ways where the magnetic forces of the atoms point in many, random directions. The forces cancel each other out. There are some special materials, though, where the atoms align in a way where the magnetic forces of most of the atoms are pointed in the same direction. The forces of the atoms combine and the object behaves as a magnet. Perpetual Motion.
Carbon Atom - Nitrogen - Oxygen - Photons (light)
Superatom is any cluster of atoms that seem to exhibit some of the properties of elemental atoms. Sodium atoms, when cooled from vapor, naturally condense into clusters, preferentially containing a magic number of atoms (2, 8, 20, 40, 58, etc.). The first two of these can be recognized as the numbers of electrons needed to fill the first and second shells, respectively. The superatom suggestion is that free electrons in the cluster occupy a new set of orbitals that are defined by the entire group of atoms, i.e. cluster, rather than each individual atom separately (non-spherical or doped clusters show deviations in the number of electrons that form a closed shell as the potential is defined by the shape of the positive nuclei.) Superatoms tend to behave chemically in a way that will allow them to have a closed shell of electrons, in this new counting scheme. Therefore, a superatom with one more electron than a full shell should give up that electron very easily, similar to an alkali metal, and a cluster with one electron short of full shell should have a large electron affinity, such as a halogen.
Waves - Resonance - Oscillation
Atomic Theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. Artificial Atom.
Atomism is a natural philosophy that developed in several ancient traditions. The atomists theorized that nature consists of two fundamental principles: Atom and Void. Unlike their modern scientific namesake in atomic theory, philosophical atoms come in an infinite variety of shapes and sizes, each indestructible, immutable and surrounded by a void where they collide with the others or hook together forming a cluster. Clusters of different shapes, arrangements, and positions give rise to the various macroscopic substances in the world.
Sound of an Atom - D-Note - 587.33 Hz (youtube)
Everything that you see in the world, including yourself, is made of just three particles of matter, protons, neutrons and electrons, that are interacting through a handful of forces, gravity, electromagnetism and the nuclear forces. DNA - Life.
An Atom is so much smaller than the wavelength of visible light that the two don’t really interact. An Atom is invisible to light itself. Even the most powerful light-focusing microscopes can’t visualize single atoms.
Atoms in your body are 99.9% empty space and none of them are the ones that you were born with. So why do I feel solid? Elementary particles have mass and the space between elementary particles is filled with the binding energy that also has the properties of mass.
Atomic Nuclei - Binding Energy
Nuclear Binding Energy is the energy that would be required to disassemble the nucleus of an atom into its component parts. These component parts are neutrons and protons, which are collectively called nucleons. The binding energy of nuclei is due to the attractive forces that hold these nucleons together, and it is always a positive number, since all nuclei would require the expenditure of energy to separate them into individual protons and neutrons. The mass of an atomic nucleus is less than the sum of the individual masses of the free constituent protons and neutrons (according to Einstein's equation E=mc2) and this 'missing mass' is known as the mass defect, and represents the energy that was released when the nucleus was formed.
Binding Energy is the energy required to disassemble a whole system into separate parts. A bound system typically has a lower potential energy than the sum of its constituent parts; this is what keeps the system together. Often this means that energy is released upon the creation of a bound state. This definition corresponds to a positive binding energy. Coulomb's Law (static) - Chemical Bonds.
Nuclear Force is the force between protons and neutrons, subatomic particles that are collectively called nucleons. The nuclear force is responsible for binding protons and neutrons into atomic nuclei. Neutrons and protons are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience a strong electric field repulsion (following Coulomb's law) that tends to push them apart, but at short range the attractive nuclear force overcomes the repulsive electromagnetic force. The mass of a nucleus is less than the sum total of the individual masses of the protons and neutrons which form it. The difference in mass between bound and unbound nucleons is known as the mass defect. Energy is released when some large nuclei break apart, and it is this energy that is used in nuclear power and nuclear weapons. (m=138 MeV) Excited State.
Atomic Nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. An atom is composed of a positively-charged nucleus, with a cloud of negatively-charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of 1.75 fm(1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen). Atom Nucleus is spherical, oblate and prolate simultaneously. Prolate is an elongated spheroid, shaped like an American football or rugby ball.
Nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines an isotope's mass number (nucleon number).
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang Nucleosynthesis (wiki).
Neutron Capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays an important role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e. by nuclear fusion), but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares.
R-Process or rapid neutron-capture process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron; the "heavy elements", with the other half produced by the p-process and s-process. The r-process usually synthesizes the most neutron-rich stable isotopes of each heavy element. The r-process can typically synthesize the heaviest four isotopes of every heavy element, and the two heaviest isotopes, which are referred to as r-only nuclei, can be created via the r-process only. Abundance peaks for the r-process occur near mass numbers A = 82 (elements Se, Br, and Kr), A = 130 (elements Te, I, and Xe) and A = 196 (elements Os, Ir, and Pt).
Why Neutrons and Protons are modified inside Nuclei. The structure of a neutron or a proton is modified when the particle is bound in an atomic nucleus. Experimental data suggest an explanation for this phenomenon that could have broad implications for nuclear physics. Modified structure of protons and neutrons in correlated pairs. The atomic nucleus is made of protons and neutrons (nucleons), which are themselves composed of quarks and gluons. Understanding how the quark–gluon structure of a nucleon bound in an atomic nucleus is modified by the surrounding nucleons is an outstanding challenge.
A careful re-analysis of data taken as revealed a possible link between correlated protons and neutrons in the nucleus and a 35-year-old mystery. The data have led to the extraction of a universal function that describes the EMC Effect, the once-shocking discovery that quarks inside nuclei have lower average momenta than predicted, and supports an explanation for the effect.
EMC Effect is the surprising observation that the cross section for deep inelastic scattering from an atomic nucleus is different from that of the same number of free protons and neutrons (collectively referred to as nucleons). From this observation, it can be inferred that the quark momentum distributions in nucleons bound inside nuclei are different from those of free nucleons. This effect was first observed in 1983 at CERN by the European Muon Collaboration, hence the name "EMC effect". It was unexpected, since the average binding energy of protons and neutrons inside nuclei is insignificant when compared to the energy transferred in deep inelastic scattering reactions that probe quark distributions. While over 1000 scientific papers have been written on the topic and numerous hypotheses have been proposed, no definitive explanation for the cause of the effect has been confirmed. Determining the origin of the EMC effect is one of the major unsolved problems in the field of nuclear physics.
Orbital Hybridisation is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridisation are in fact not related to the VSEPR model.
Stable Atom is an atom that has enough binding energy to hold the nucleus together permanently. An unstable atom does not have enough binding energy to hold the nucleus together permanently and is called a radioactive atom.
Island of Stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclides, separated from known stable and long-lived primordial radionuclides. Its theoretical existence is attributed to stabilizing effects of predicted "magic numbers" of protons and neutrons in the superheavy mass region.
Brownian Motion is the random motion of particles suspended in a fluid (a liquid or a gas) resulting from their collision with the fast-moving atoms or molecules in the gas or liquid. Elements.
Breakthrough in Nuclear Physics. High-precision measurements of the strong interaction between stable and unstable particles. The positively charged protons in atomic nuclei should actually repel each other, and yet even heavy nuclei with many protons and neutrons stick together. The so-called strong interaction is responsible for this. Scientists have now developed a method to precisely measure the strong interaction utilizing particle collisions in the ALICE experiment at CERN in Geneva. The strong interaction is one of the four fundamental forces in physics. It is essentially responsible for the existence of atomic nuclei that consist of several protons and neutrons. Protons and neutrons are made up of smaller particles, the so-called quarks. And they too are held together by the strong interaction. ALICE stands for A Large Ion Collider Experiment.
Proton
Proton is a subatomic particle, symbol p or p+, with a positive electric charge of +1e elementary charge and mass slightly less than that of a neutron. Protons and neutrons, each with masses of approximately one atomic mass unit, are collectively referred to as "nucleons". One or more protons are present in the nucleus of every atom. They are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol Z). Antiproton is the antiparticle of the proton. Antiprotons are stable, but they are typically short-lived, since any collision with a proton will cause both particles to be annihilated in a burst of energy. Protons are composite particles composed of three valence quarks: two up quarks of charge +23e and one down quark of charge –13e. In vacuum, when free electrons are present, a sufficiently slow proton may pick up a single free electron, becoming a neutral hydrogen atom, which is chemically a free radical.
Charge Radius is a measure of the size of an atomic nucleus, particularly of a proton or a deuteron. It can be measured by the scattering of electrons by the nucleus and also inferred from the effects of finite nuclear size on electron energy levels as measured in atomic spectra.
Proton Radius Puzzle was an unanswered problem in physics relating to the size of the proton. Historically the proton radius was measured via two independent methods, which converged to a value of about 0.877 femtometres (1 fm = 10−15 m). This value was challenged by a 2010 experiment utilizing a third method, which produced a radius about 5% smaller than this, or 0.842 femtometres. The discrepancy was resolved when research conducted by Hessel et al. confirmed the same radius for 'electronic' hydrogen as well as its 'muonic' variant. (0.833 fentometers).
Interaction of Free Protons with ordinary Matter. Although protons have affinity for oppositely charged electrons, this is a relatively low-energy interaction and so free protons must lose sufficient velocity (and kinetic energy) in order to become closely associated and bound to electrons. High energy protons, in traversing ordinary matter, lose energy by collisions with atomic nuclei, and by ionization of atoms or removing electrons until they are slowed sufficiently to be captured by the electron cloud in a normal atom. However, in such an association with an electron, the character of the bound proton is not changed, and it remains a proton. The attraction of low-energy free protons to any electrons present in normal matter (such as the electrons in normal atoms) causes free protons to stop and to form a new chemical bond with an atom. Such a bond happens at any sufficiently "cold" temperature (i.e., comparable to temperatures at the surface of the Sun) and with any type of atom. Thus, in interaction with any type of normal (non-plasma) matter, low-velocity free protons are attracted to electrons in any atom or molecule with which they come in contact, causing the proton and molecule to combine. Such molecules are then said to be "protonated", and chemically they often, as a result, become so-called Brønsted acids.
Proton Pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Transport of the positively charged proton is typically electrogenic, i.e. it generates an electrical field across the membrane also called the membrane potential. Proton transport becomes electrogenic if not neutralized electrically by transport of either a corresponding negative charge in the same direction or a corresponding positive charge in the opposite direction. An example of a proton pump that is not electrogenic, is the proton/potassium pump of the gastric mucosa which catalyzes a balanced exchange of protons and potassium ions. The combined transmembrane gradient of protons and charges created by proton pumps is called an electrochemical gradient. An electrochemical gradient represents a store of energy or potential energy that can be used to drive a multitude of biological processes such as ATP synthesis, nutrient uptake and action potential formation. In cell respiration, the proton pump uses energy to transport protons from the matrix of the mitochondrion to the inter-membrane space. It is an active pump that generates a proton concentration gradient across the inner mitochondrial membrane because there are more protons outside the matrix than inside. The difference in pH and electric charge (ignoring differences in buffer capacity) creates an electrochemical potential difference that works similar to that of a battery or energy storing unit for the cell. The process could also be seen as analogous to cycling uphill or charging a battery for later use, as it produces potential energy. The proton pump does not create energy, but forms a gradient that stores energy for later use. Proton Tunneling.
Neutron
Neutron is a subatomic particle, symbol n or n0, with no net electric charge. The amount of positive and negative charges in the neutron are equal. So an electrically neutral object does contain charges. The neutron mass slightly larger than that of a proton. Protons and neutrons, each with mass approximately one atomic mass unit, constitute the nucleus of an atom, and they are collectively referred to as nucleons. Their properties and interactions are described by nuclear physics. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element that differ only in neutron number are called isotopes. For example, carbon, with atomic number 6, has an abundant isotope carbon-12 with 6 neutrons and a rare isotope carbon-13 with 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine. Other elements occur with many stable isotopes, such as tin with ten stable isotopes. The properties of an atomic nucleus depend on both atomic and neutron numbers. With their positive charge, the protons within the nucleus are repelled by the long-range electromagnetic force, but the much stronger, but short-range, nuclear force binds the nucleons closely together. Neutrons are required for the stability of nuclei, with the exception of the single-proton hydrogen nucleus. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered by James Chadwick in 1932, neutrons were used to induce many different types of nuclear transmutations. With the discovery of nuclear fission in 1938, it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, in a cascade known as a nuclear chain reaction. These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945). Free neutrons, while not directly ionizing atoms, cause ionizing radiation. So they can be a biological hazard, depending on dose. A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust. Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments.
Isotopes are variants of a particular chemical element which differ in neutron number. All isotopes of a given element have the same number of protons in each atom. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number. For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element Carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.
Electron
 Electron is a
subatomic particle, symbol e−orβ−, with a
negative
electric
charge with an electric field. An electron
moving in orbit creates a magnetic field with a
magnetic monopole that does not require
a positive charge. Electrons belong to the first generation of the lepton
particle
family, and are generally thought to be elementary particles because they
have no known components or substructure. The electron has a mass that is
approximately 1/1836 that of the proton. An electron can
spin up or spin down. Quantum mechanical properties of
the electron include an intrinsic
angular momentum
or spin of a
half-integer value, expressed in units of the reduced Planck constant, ħ.
As it is a fermion, no two electrons can occupy the same quantum state, in
accordance with the pauli exclusion principle. Like all matter, electrons
have properties of both particles and waves: they can collide with other
particles and can be diffracted like light. The wave properties of
electrons are easier to observe with experiments than those of other
particles like neutrons and protons because electrons have a lower mass
and hence a larger De Broglie wavelength for a given energy.
Poly-electron is an atom containing more
than one electron. Hydrogen is the only atom in the periodic table that
has one electron in the orbitals under ground state.
Electron is a
subatomic particle, symbol e−orβ−, with a
negative
electric
charge with an electric field. An electron
moving in orbit creates a magnetic field with a
magnetic monopole that does not require
a positive charge. Electrons belong to the first generation of the lepton
particle
family, and are generally thought to be elementary particles because they
have no known components or substructure. The electron has a mass that is
approximately 1/1836 that of the proton. An electron can
spin up or spin down. Quantum mechanical properties of
the electron include an intrinsic
angular momentum
or spin of a
half-integer value, expressed in units of the reduced Planck constant, ħ.
As it is a fermion, no two electrons can occupy the same quantum state, in
accordance with the pauli exclusion principle. Like all matter, electrons
have properties of both particles and waves: they can collide with other
particles and can be diffracted like light. The wave properties of
electrons are easier to observe with experiments than those of other
particles like neutrons and protons because electrons have a lower mass
and hence a larger De Broglie wavelength for a given energy.
Poly-electron is an atom containing more
than one electron. Hydrogen is the only atom in the periodic table that
has one electron in the orbitals under ground state.A new window into electron behavior. Quantum mechanical tunneling material's band structure. Momentum and energy is a process by which electrons can traverse energetic barriers by simply appearing on the other side. An electron is a wave of probability. Q-Bit - Entanglement.
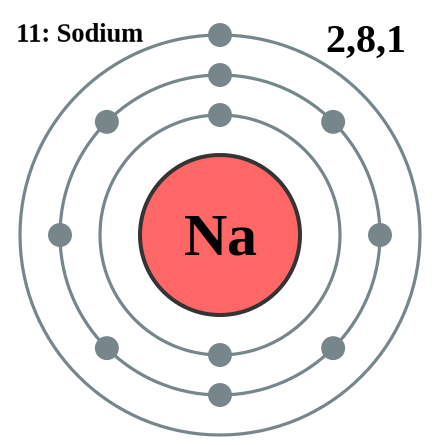
Electron Shell is the area that electrons orbit in around an atom's nucleus. Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. Shell 1 can hold up to 2 electrons, Shell 2 can hold up to 8 electrons, Shell 3 can hold up to 18 electrons, Shell 4 can hold up to 32 electrons, Shell 5 can hold up to 50 electrons. The general formula is that the nth shell can in principle hold up to 2(n2) electrons. Since electrons are electrically attracted to the nucleus, an atom's electrons will generally occupy outer shells only if the more inner shells have already been completely filled by other electrons. However, this is not a strict requirement: atoms may have two or even three incomplete outer shells. The valence shell is the outermost shell of an atom in its uncombined state, which contains the electrons most likely to account for the nature of any reactions involving the atom and of the bonding interactions it has with other atoms. Care must be taken to note that the outermost shell of an ion is not commonly termed valence shell. Electrons in the valence shell are referred to as valence electrons. Energy Level of a particle that is bound or confined spatially—can only take on certain discrete values of energy. If the potential energy is set to zero at infinite distance from the atomic nucleus or molecule, the usual convention, then bound electron states have negative potential energy. If an atom, ion, or molecule is at the lowest possible energy level, it and its electrons are said to be in the ground state. If it is at a higher energy level, it is said to be excited, or any electrons that have higher energy than the ground state are excited. If more than one quantum mechanical state is at the same energy, the energy levels are "degenerate". They are then called degenerate energy levels. (electrons also travel through protons).
Sublevels of Electron are known by the letters s, p, d, and f. The s sublevel has just one orbital, so can contain 2 electrons max. The p sublevel has 3 orbitals, so can contain 6 electrons max. The d sublevel has 5 orbitals, so can contain 10 electrons max. And the 4 sublevel has 7 orbitals, so can contain 14 electrons max. Examples of the subevels found in various atoms are shown below. The superscript shows the number of electrons in each sublevel. Hydrogen: 1s1, Carbon: 1s2 2s2 2p2, Chlorine: 1s2 2s2 2p6 3s2 3p5, Argon: 1s2 2s2 2p6 3s2 3p6. The sublevels contain orbitals. The s sublevel has just one orbital, so can contain 2 electrons max. The p sublevel has 3 orbitals, so can contain 6 electrons max. The d sublevel has 5 orbitals, so can contain 10 electrons max.
Atomic Orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term atomic orbital may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.
Electron Spin is a quantum property of electrons. It is a form of angular momentum. The magnitude of this angular momentum is permanent. Like charge and rest mass, spin is a fundamental, unvarying property of the electron. If the electron spins clockwise on its axis, it is described as spin-up; counterclockwise is spin-down. This is a convenient explanation, if not fully justifiable mathematically. The spin angular momentum associated with electron spin is independent of orbital angular momentum, which is associated with the electron's journey around the nucleus. Electron spin is not used to define electron shells, subshells, or orbitals, unlike the quantum numbers n, l, and ml. Since these two electrons are in the same orbital, they occupy the same region of space within the atom. As a result, their spin quantum numbers cannot be the same, and thus these two electrons cannot exist in the same atom.
Spintronics is the study of the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid-state devices. Spintronics fundamentally differs from traditional electronics in that, in addition to charge state, electron spins are exploited as a further degree of freedom, with implications in the efficiency of data storage and transfer. Spintronic systems are most often realised in dilute magnetic semiconductors (DMS) and Heusler alloys and are of particular interest in the field of quantum computing.
Valence Electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can exist only in the outermost electron shell. In a transition metal, a valence electron can also be in an inner shell. Redox.
The electron can gain the energy it needs by absorbing light. If the electron jumps from the second energy level down to the first energy level, it must give off some energy by emitting light. The atom absorbs or emits light in discrete packets called photons, and each photon has a definite energy. Kinetic and potential energy of atoms result from the motion of electrons. When electrons are excited they move to a higher energy orbital farther away from the atom. The further the orbital is from the nucleus, the higher the potential energy of an electron at that energy level.
Cathode Ray are streams of electrons observed in vacuum tubes that were first observed in 1869. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from and traveling away from the cathode (the electrode connected to the negative terminal of the voltage supply). Cathode Ray Tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to create the image on a television screen. Particle Accelerator.
Excited State of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being raised to an excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often called metastable. Long-lived nuclear isomers and singlet oxygen are two examples of this.
Hot Electrons are electrons that have gained very high levels of kinetic energy after being accelerated by a strong electric field in areas of high field intensities within a semiconductor. (a type of 'hot carriers').
Hot-Carrier Injection is a phenomenon in solid-state electronic devices where an electron or a “hole” gains sufficient kinetic energy to overcome a potential barrier necessary to break an interface state. The term "hot" refers to the effective temperature used to model carrier density, not to the overall temperature of the device. Since the charge carriers can become trapped in the gate dielectric of a MOS transistor, the switching characteristics of the transistor can be permanently changed. Hot-carrier injection is one of the mechanisms that adversely affects the reliability of semiconductors of solid-state devices.
Valence and Conduction Bands. The band of energy occupied by the valence electrons is called the valence band. The valence band is the highest occupied band. Conduction Band:-The conduction band is normally empty and may be defined as the lowest unfilled energy band. In the conduction band, electrons can move freely and are generally called conduction electrons.
Electron Paramagnetic Resonance is a method for studying materials with unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of the spins of atomic nuclei.
The geometry of an electron determined for the first time.
Positron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1 e, a spin of 1/2 (same as electron), and has the same mass as an electron. When a positron collides with an electron, annihilation occurs. If this collision occurs at low energies, it results in the production of two or more gamma ray photons Positrons may be generated by positron emission radioactive decay (through weak interactions), or by pair production from a sufficiently energetic photon which is interacting with an atom in a material.
Valleytronics refers to the technology of control over the valley degree of freedom (a local maximum/minimum on the valence/conduction band) of certain semiconductors that present multiple valleys inside the first Brillouin zone—known as multivalley semiconductors. The term was coined in analogy to the blooming field of spintronics. While in spintronics the internal degree of freedom of spin is harnessed to store, manipulate and read out bits of information, the proposal for valleytronics is to perform similar tasks using the multiple extrema of the band structure, so that the information of 0s and 1s would be stored as different discrete values of the crystal momentum.
Mechanical Vibration generated by Electron Spins. A new way to deliver a force to drive micro mechanics.
A multiband approach to Coulomb drag and indirect excitons in which coupled charged particles moved in exactly the opposite direction to that predicted. This apparently contradictory phenomenon is associated with the bandgap in dual-layer graphene structures, a bandgap which is very much smaller than in conventional semiconductors.
Transition Metal is an element whose atom has a partially filled d sub-shell, or which can give rise to cations with an incomplete d sub-shell". Or any element in the d-block of the periodic table, which includes groups 3 to 12 on the periodic table. In actual practice, the f-block lanthanide and actinide series are also considered transition metals and are called "inner transition metals".
Semimetal is a material with a very small overlap between the bottom of the conduction band and the top of the valence band. According to electronic band theory, solids can be classified as insulators, semiconductors, semimetals, or metals. In insulators and semiconductors the filled valence band is separated from an empty conduction band by a band gap.
Electron Configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
Physicists discover topological behavior of electrons in 3D magnetic material. An international team of researchers led by scientists at Princeton University has found that a magnetic material at room temperature enables electrons to behave counterintuitively, acting collectively rather than as individuals. Their collective behavior mimics massless particles and anti-particles that coexist in an unexpected way and together form an exotic loop-like structure. Researchers explored a type of material in which the electrons behave according to the mathematical rules of topology. They found topological behaviors of electrons in a three-dimensional magnetic material at room temperature, opening new avenues of future study. The key to this behavior is topology -- a branch of mathematics that is already known to play a powerful role in dictating the behavior of electrons in crystals. Topological materials can contain massless particles in the form of light, or photons. In a topological crystal, the electrons often behave like slowed-down light yet, unlike light, carry electrical charge. Topology has seldom been observed in magnetic materials, and the finding of a magnetic topological material at room temperature is a step forward that could unlock new approaches to harnessing topological materials for future technological applications. The exotic magnetic crystal consists of cobalt, manganese and gallium, arranged in an orderly, repeating three-dimensional pattern. To explore the material's topological state, the researchers used a technique called angle-resolved photoemission spectroscopy. In this experiment, high-intensity light shines on the sample, forcing electrons to emit from the surface. These emitted electrons can then be measured, providing information about the way the electrons behaved when they were inside the crystal.
Electron Sharing
Electron Transfer occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of a redox reaction, wherein the oxidation state of reactant and product changes. Mitochondria.
Electron Donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. Ph.
Electron Acceptor a complete and irreversible transfer of one or more electrons, not completely transferred, but results in an electron resonance between the donor and acceptor. The microbes aren’t eating naked electrons. Electrons traverse the entire distance of the membrane unescorted.
Reduction Potential - Oxidation - Unpaired Electron - Grounding
Electron Pair consists of two electrons that occupy the same molecular orbital but have opposite spins.
Unpaired Electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain two electrons (electron pair) with opposite spins. As the formation of electron pairs is often energetically favourable, either in the form of a chemical bond or as a lone pair, unpaired electrons are relatively uncommon in chemistry, because an entity that carries an unpaired electron is usually rather reactive. In organic chemistry they typically only occur briefly during a reaction on an entity called a radical; however, they play an important role in explaining reaction pathways. Radicals are uncommon in s- and p-block chemistry, since the unpaired electron occupies a valence p orbital or an sp, sp2 or sp3 hybrid orbital. These orbitals are strongly directional and therefore overlap to form strong covalent bonds, favouring dimerisation of radicals. Radicals can be stable if dimerisation would result in a weak bond or the unpaired electrons are stabilised by delocalisation. In contrast, radicals in d- and f-block chemistry are very common. The less directional, more diffuse d and f orbitals, in which unpaired electrons reside, overlap less effectively, form weaker bonds and thus dimerisation is generally disfavoured. These d and f orbitals also have comparatively smaller radial extension, disfavouring overlap to form dimers. Relatively more stable entities with unpaired electrons do exist, e.g. the nitric oxide molecule has one. According to Hund's rule, the spins of unpaired electrons are aligned parallel and this gives these molecules paramagnetic properties. The most stable examples of unpaired electrons are found on the atoms and ions of lanthanides and actinides. The incomplete f-shell of these entities does not interact very strongly with the environment they are in and this prevents them from being paired. The ions with the largest number of unpaired electrons are Gd3+ and Cm3+ with seven unpaired electrons. An unpaired electron has a magnetic dipole moment, while an electron pair has no dipole moment because the two electrons have opposite spins so their magnetic dipole fields are in opposite directions and cancel. Thus an atom with unpaired electrons acts as a magnetic dipole and interacts with a magnetic field. Only elements with unpaired electrons exhibit paramagnetism, ferromagnetism, and antiferromagnetism.
Lone Pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of lone pair electrons plus the number of bonding electrons equals the total number of valence electrons around an atom.
Exciton is a bound state of an electron and an electron hole which are attracted to each other by the electrostatic Coulomb force. It is an electrically neutral quasiparticle that exists in insulators, semiconductors and in some liquids. The exciton is regarded as an elementary excitation of condensed matter that can transport energy without transporting net electric charge. An exciton can form when a photon is absorbed by a semiconductor. This excites an electron from the valence band into the conduction band. In turn, this leaves behind a positively charged electron hole (an abstraction for the location from which an electron was moved). The electron in the conduction band is then effectively attracted to this localized hole by the repulsive Coulomb forces from large numbers of electrons surrounding the hole and excited electron. This attraction provides a stabilizing energy balance. Consequently, the exciton has slightly less energy than the unbound electron and hole. The wavefunction of the bound state is said to be hydrogenic, an exotic atom state akin to that of a hydrogen atom. However, the binding energy is much smaller and the particle's size much larger than a hydrogen atom. This is because of both the screening of the Coulomb force by other electrons in the semiconductor (i.e., its dielectric constant), and the small effective masses of the excited electron and hole. The recombination of the electron and hole, i.e. the decay of the exciton, is limited by resonance stabilization due to the overlap of the electron and hole wave functions, resulting in an extended lifetime for the exciton.
Quantized Energy states that electrons are necessary for atoms to exist. But where does an electron get its energy from? How do electrons circulate around the nucleus forever in perpetual motion? Is it electromagnetic radiation or God?. Is it true that Atoms do not have to get energy from somewhere, because they are energy? Einstein proposed that mass and energy are two sides of the same coin. Mass can convert into energy and vice-versa. In reality, all matter we see is a manifestation of energy. Matter is nothing but hypercondensed energy, and this energy can vibrate at different frequencies, giving rise to fundamental forces based on the vibrational patterns. This is what the string theory describes as a "String". Thus we see that matter itself is energy. However in chemical reactions, it may use its own internal energy, or absorb energy from surroundings in the form of heat, light etc.. What came first, the chicken or the egg?
Quantum Mechanics tells us that electrons have both wave and particle-like properties.
Tunneling is a quantum mechanical effect. A tunneling current occurs when electrons move through a barrier that they classically shouldn't be able to move through. In classical terms, if you don't have enough energy to move "over" a barrier, you won't. However, in the quantum mechanical world, electrons have wavelike properties. These waves don’t end abruptly at a wall or barrier, but taper off quickly. If the barrier is thin enough, the probability function may extend into the next region, through the barrier! Because of the small probability of an electron being on the other side of the barrier, given enough electrons, some will indeed move through and appear on the other side. When an electron moves through the barrier in this fashion, it is called tunneling.
Anti-Hydrogen is the antimatter counterpart of Hydrogen. Whereas the common Hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. Scientists hope studying antihydrogen may shed light on the question of why there is more matter than antimatter in the universe, known as the baryon asymmetry problem. Antihydrogen is produced artificially in particle accelerators. In 1999, NASA gave a cost estimate of $62.5 trillion per gram of antihydrogen (equivalent to $90 trillion today), making it the most expensive material to produce. This is due to the extremely low yield per experiment, and high opportunity cost of using a particle accelerator.
Machine learning reveals how strongly interacting electrons behave at atomic level.
Electron Transport Chain is a series of complexes that transfer electrons from electron donors to electron acceptors via redox (both reduction and oxidation occurring simultaneously) reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electron transport chain is built up of peptides, enzymes, and other molecules. A series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water.
Photoelectric Effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission. In classical electromagnetic theory, the photoelectric effect would be attributed to the transfer of energy from the continuous light waves to an electron. An alteration in the intensity of light would change the kinetic energy of the emitted electrons, and sufficiently dim light would result in the emission delayed by the time it would take the electrons to accumulate enough energy to leave the material. The experimental results, however, disagree with both predictions. Instead, they show that electrons are dislodged only when the light exceeds a threshold frequency. Below that threshold, no electrons are emitted from the material, regardless of the light intensity or the length of time of exposure to the light. Because a low-frequency beam at a high intensity could not build up the energy required to produce photoelectrons like it would have if light's energy was coming from a continuous wave, Einstein proposed that a beam of light is not a wave propagating through space, but a collection of discrete wave packets—photons. Emission of conduction electrons from typical metals requires a few electron-volt (eV) light quanta, corresponding to short-wavelength visible or ultraviolet light. In extreme cases, emissions are induced with photons approaching zero energy, like in systems with negative electron affinity and the emission from excited states, or a few hundred keV photons for core electrons in elements with a high atomic number. Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality. Other phenomena where light affects the movement of electric charges include the photoconductive effect, the photovoltaic effect, and the photoelectrochemical effect.
Ions - Ionization
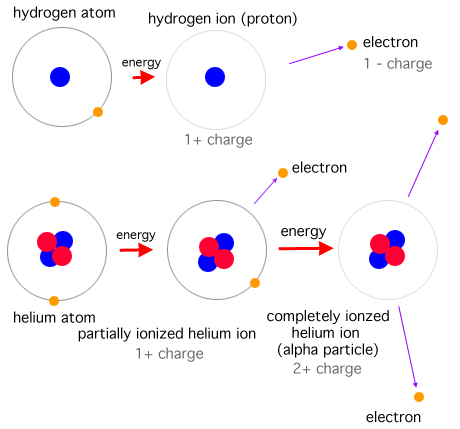 Ion is an atom or a
molecule in which the total number of
electrons is not equal to the total
number of protons, giving the atom or molecule a net
positive or negative electrical
charge. Positive ions are
molecules that have lost one or more electrons whereas negative ions are
atoms with extra-negatively-charged electrons. An ion is
an atom or a group of atoms where the number of electrons is not equal to
the number of protons. An ion can also be an atom without any electrons. Lasers.
Ion is an atom or a
molecule in which the total number of
electrons is not equal to the total
number of protons, giving the atom or molecule a net
positive or negative electrical
charge. Positive ions are
molecules that have lost one or more electrons whereas negative ions are
atoms with extra-negatively-charged electrons. An ion is
an atom or a group of atoms where the number of electrons is not equal to
the number of protons. An ion can also be an atom without any electrons. Lasers.Negative ions can improve mood and are believed to produce biochemical reactions that increase levels of the mood chemical serotonin. Water Falls are a great source of negative ions, so go outside. Avoid ion air filters that create ozone. Grounding - Being in Nature Benefits.
Cation is a positively charged ion. Ions can be created, by either chemical or physical means, via ionization.
Anion is a negatively charged ion. Atoms or radicals groups of atoms, that have gained electrons, now have more electrons than protons, thus anions have a negative charge.
Ion Implantation is a low-temperature process by which ions of one element are accelerated into a solid target, thereby changing the physical, chemical, or electrical properties of the target. Ion implantation is used in semiconductor device fabrication and in metal finishing, as well as in materials science research. The ions can alter the elemental composition of the target (if the ions differ in composition from the target) if they stop and remain in the target. Ion implantation also causes chemical and physical changes when the ions impinge on the target at high energy. The crystal structure of the target can be damaged or even destroyed by the energetic collision cascades, and ions of sufficiently high energy (10s of MeV) can cause nuclear transmutation. Inside smartphones are chips that are made by implanting single ions into silicon, in a process called ion implantation. And that uses a particle accelerator.
Polyatomic Ion is a charged chemical species (ion) composed of two or more atoms covalently bonded or of a metal complex that can be considered to be acting as a single unit. The prefix poly- means "many," in Greek, but even ions of two atoms are commonly referred to as polyatomic. In older literature, a polyatomic ion is also referred to as a radical, and less commonly, as a radical group. In contemporary usage, the term radical refers to free radicals that are (not necessarily charged) species with an unpaired electron.
Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Ionizing Radiation is radiation that carries enough energy to free electrons from atoms or molecules, thereby ionizing them. Ionizing radiation is made up of energetic subatomic particles, ions or atoms moving at high speeds (usually greater than 1% of the speed of light), and electromagnetic waves on the high-energy end of the electromagnetic spectrum. Three ways to remove an electron from an atom: 1) Annihilate the electron by hitting the target (atom, ion, molecule, etc.) with a positron. 2) Hit the target with a very fast electron or high energy x-ray, shooting the electron off into space. 3) Transfer the electron to another atom, ion, molecule etc. (reduction oxidation chemistry). The electron leaves the atom when its kinetic energy is higher than the attraction between it and the nucleus. Binding Energy - Hydrogen.
Ionizing Radiation is any type of particle or electromagnetic wave that carries enough energy to ionize or remove electrons from an atom. There are two types of electromagnetic waves that can ionize atoms: X-rays and gamma-rays, and sometimes they have the same energy. Ionizing Radiation is radiation that carries enough energy to knock electrons from atoms or molecules, thereby ionizing them. Ionizing radiation is made up of energetic subatomic particles, ions or atoms moving at high speeds (usually greater than 1% of the speed of light), and electromagnetic waves on the high-energy end of the electromagnetic spectrum. Radio Active Decay - Extremophile.
Non-Ionizing Radiation refers to any type of electromagnetic radiation that does not carry enough energy per quantum (photon energy) to ionize atoms or molecules—that is, to completely remove an electron from an atom or molecule. Instead of producing charged ions when passing through matter, non-ionizing electromagnetic radiation has sufficient energy only for excitation, the movement of an electron to a higher energy state. Ionizing radiation which has a higher frequency and shorter wavelength than nonionizing radiation, has many uses but can be a health hazard; exposure to it can cause burns, radiation sickness, cancer, and genetic damage. Using ionizing radiation requires elaborate radiological protection measures which in general are not required with nonionizing radiation.
Redox
Reduction Potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts (V), or millivolts (mV). Each species has its own intrinsic reduction potential; the more positive the potential, the greater the species' affinity for electrons and tendency to be reduced. ORP is a common measurement for water quality. (also known as redox potential, oxidation).
Redox is a chemical reaction in which the oxidation states of atoms are changed. Any such reaction involves both a reduction process and a complementary oxidation process, two key concepts involved with electron transfer processes. Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between chemical species. The chemical species from which the electron is stripped is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. It can be explained in simple terms: Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion. As an example, during the combustion of wood, oxygen from the air is reduced, gaining electrons from carbon which is oxidized. Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, oxygen is not necessarily included in such reactions, as other chemical species can serve the same function. The reaction can occur relatively slowly, as with the formation of rust, or more quickly, in the case of fire. There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body. Redox is a contraction of the name for a chemical reduction–oxidation reaction. Any such reaction involves both a reduction process and a complementary oxidation process. Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between chemical species. Antioxidant.
Oxidation is any chemical reaction that involves the moving of electrons. Specifically, it means the substance that gives away electrons is oxidized. When iron reacts with oxygen it forms a chemical called rust because it has been oxidized (The iron has lost some electrons.) and the oxygen has been reduced (The oxygen has gained some electrons.). Oxidation is the opposite of reduction. A reduction-reaction always comes together with an oxidation-reaction. Oxidation and reduction together are called redox (reduction and oxidation). Oxygen does not have to be present in a reaction, for it to be a redox-reaction. Oxidation is the loss of electrons.
Oxidizing Agent is a substance that has the ability to oxidize other substances, in other words to cause them to lose electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.
Ozone is a powerful oxidant, far more so than dioxygen, and has many industrial and consumer applications related to oxidation. This same high oxidising potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about 0.1 ppm. While this makes ozone a potent respiratory hazard and pollutant near ground level, a higher concentration in the ozone layer (from two to eight ppm) is beneficial, preventing damaging UV light from reaching the Earth's surface. Ozone or trioxygen, is an inorganic molecule with the chemical formula O3. It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O2, breaking down in the lower atmosphere to O2 (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet light (UV) and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the latter, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.
Electronegativity is a chemical property that describes the tendency of an atom to attract a shared pair of electrons (or electron density) towards itself. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number/location of other electrons present in the atomic shells (the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result the less positive charge they will experience—both because of their increased distance from the nucleus, and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus). The opposite of electronegativity is Electropositivity is a measure of an element's ability to donate electrons.
"We have just worked out what atoms are, and we’ve realized that they are marvelously complex structures that can undergo amazing changes, many of which occur naturally. And by studying atoms this way, we’ve been able to improve our technologies, harness the energy of nuclear reactions and better understand the natural world around us. We’ve also been able to better protect ourselves from radiation and discover how materials change when placed under extreme conditions."
Particles
Particle is a tiny piece of anything having finite mass and internal structure but negligible dimensions.
Particle is a minute fragment or quantity of matter. In the physical sciences, a particle is a small localized object to which can be ascribed several physical or chemical properties such as volume or mass. They vary greatly in size, from subatomic particles like the electron, to microscopic particles like atoms and molecules, to macroscopic particles like powders and other granular materials. Particles can also be used to create scientific models of even larger objects, such as humans moving in a crowd or celestial bodies in motion.
Wave - Quantum - Binding Energy - Particle Detectors
Massless Particle is an elementary particle whose invariant mass is zero. The two known massless particles are both gauge bosons: the photon (carrier of electromagnetism) and the gluon (carrier of the strong force). However, gluons are never observed as free particles, since they are confined within hadrons. Neutrinos were originally thought to be massless. However, because neutrinos change flavor as they travel, at least two of the types of neutrinos must have mass. Neutrino Oscillation.
List of Particles (wiki) - Photons (light) - Electrons
Particles of the Standard Model (image)
Particle Adventure - Particle Smashing (partical accelerator)
Massive Particle refers to particles which have real non-zero rest mass. According to special relativity, their velocity is always lower than the speed of light. The synonyms bradyon (from Greek: βραδύς, bradys, “slow”), tardyon or ittyon are sometimes used to contrast with luxon (which moves at light speed) and hypothetical tachyon (which moves faster than light).
Charged Particle is a particle with an electric charge. It may be an ion, such as a molecule or atom with a surplus or deficit of electrons relative to protons. It can be the electrons and protons themselves, as well as other elementary particles, like positrons. It may also be an atomic nucleus devoid of electrons, such as an alpha particle, a helium nucleus. Neutrons have no charge. Plasmas are a collection of charged particles, atomic nuclei and separated electrons, but can also be a gas containing a significant proportion of charged particles. Plasma is called the fourth state of matter because its properties are quite different from solids, liquids and gases. Aurora.
Chameleon Particle is a hypothetical scalar particle that couples to matter more weakly than gravity, postulated as a dark energy candidate. Due to a non-linear self-interaction, it has a variable effective mass which is an increasing function of the ambient energy density—as a result, the range of the force mediated by the particle is predicted to be very small in regions of high density (for example on Earth, where it is less than 1mm) but much larger in low-density intergalactic regions: out in the cosmos chameleon models permit a range of up to several thousand parsecs. As a result of this variable mass, the hypothetical fifth force mediated by the chameleon is able to evade current constraints on equivalence principle violation derived from terrestrial experiments even if it couples to matter with a strength equal or greater than that of gravity. Although this property would allow the chameleon to drive the currently observed acceleration of the universe's expansion, it also makes it very difficult to test for experimentally. Dark Energy.
Virtual Particle is a transient fluctuation that exhibits many of the characteristics of an ordinary particle, but that exists for a limited time. The concept of virtual particles arises in perturbation theory of quantum field theory where interactions between ordinary particles are described in terms of exchanges of virtual particles. Any process involving virtual particles admits a schematic representation known as a Feynman diagram, in which virtual particles are represented by internal lines.
Quasiparticle are emergent phenomena that occur when a microscopically complicated system such as a solid behaves as if it contained different weakly interacting particles in free space. For example, as an electron travels through a semiconductor, its motion is disturbed in a complex way by its interactions with all of the other electrons and nuclei; however it approximately behaves like an electron with a different mass (effective mass) traveling unperturbed through free space. This "electron with a different mass" is called an "electron quasiparticle". In another example, the aggregate motion of electrons in the valence band of a semiconductor or a hole band in a metal is the same as if the material instead contained positively charged quasiparticles called electron holes. Other quasiparticles or collective excitations include phonons (particles derived from the vibrations of atoms in a solid), plasmons (particles derived from plasma oscillations), and many others. These particles are typically called "quasiparticles" if they are related to fermions, and called "collective excitations" if they are related to bosons,[1] although the precise distinction is not universally agreed upon. Thus, electrons and electron holes are typically called "quasiparticles", while phonons and plasmons are typically called "collective excitations". The quasiparticle concept is most important in condensed matter physics since it is one of the few known ways of simplifying the quantum mechanical many-body problem.
Magnon is a quasiparticle, a collective excitation of the electrons' spin structure in a crystal lattice. In the equivalent wave picture of quantum mechanics, a magnon can be viewed as a quantized spin wave. Magnons carry a fixed amount of energy and lattice momentum, and are spin-1, indicating they obey boson behavior.
Unparticle is a speculative theory that conjectures a form of matter that cannot be explained in terms of particles using the Standard Model of particle physics, because its components are scale invariant.
Anti-Particle. Every type of particle has an associated antiparticle with the same mass but with opposite physical charges (such as electric charge). Corresponding to most kinds of particles, there is an associated antiparticle with the same mass and opposite charge (including electric charge). For example, the antiparticle of the electron is the positron (antielectron), which has positive charge and is produced naturally in certain types of radioactive decay. The opposite is also true: the antiparticle of the positron is the electron. Some particles, such as the photon, are their own antiparticle. Otherwise, for each pair of antiparticle partners, one is designated as normal matter (the kind we are made of), and the other (usually given the prefix "anti-") as in antimatter. Particle–antiparticle pairs can annihilate each other, producing photons; since the charges of the particle and antiparticle are opposite, total charge is conserved. For example, the positrons produced in natural radioactive decay quickly annihilate themselves with electrons, producing pairs of gamma rays, a process exploited in positron emission tomography. The laws of nature are very nearly symmetrical with respect to particles and antiparticles. For example, an antiproton and a positron can form an antihydrogen atom, which is believed to have the same properties as a hydrogen atom. This leads to the question of why the formation of matter after the Big Bang resulted in a universe consisting almost entirely of matter, rather than being a half-and-half mixture of matter and antimatter. The discovery of Charge Parity violation helped to shed light on this problem by showing that this symmetry, originally thought to be perfect, was only approximate. Antiparticles are produced naturally in beta decay, and in the interaction of cosmic rays in the Earth's atmosphere. Because charge is conserved, it is not possible to create an antiparticle without either destroying a particle of the same charge (as in β+ decay, when a proton (positive charge) is destroyed, a neutron created and a positron (positive charge, antiparticle) is also created and emitted) or by creating a particle of the opposite charge. The latter is seen in many processes in which both a particle and its antiparticle are created simultaneously, as in particle accelerators. This is the inverse of the particle–antiparticle annihilation process. Although particles and their antiparticles have opposite charges, electrically neutral particles need not be identical to their antiparticles. The neutron, for example, is made out of quarks, the antineutron from antiquarks, and they are distinguishable from one another because neutrons and antineutrons annihilate each other upon contact. However, other neutral particles are their own antiparticles, such as photons, Z0 bosons,0 mesons, and hypothetical gravitons and some hypothetical WIMPs. Antimatter.
Subatomic Particle are particles much smaller than atoms. There are two types of subatomic particles: elementary particles, which according to current theories are not made of other particles; and composite particles. Particle physics and nuclear physics study these particles and how they interact. Electrons.
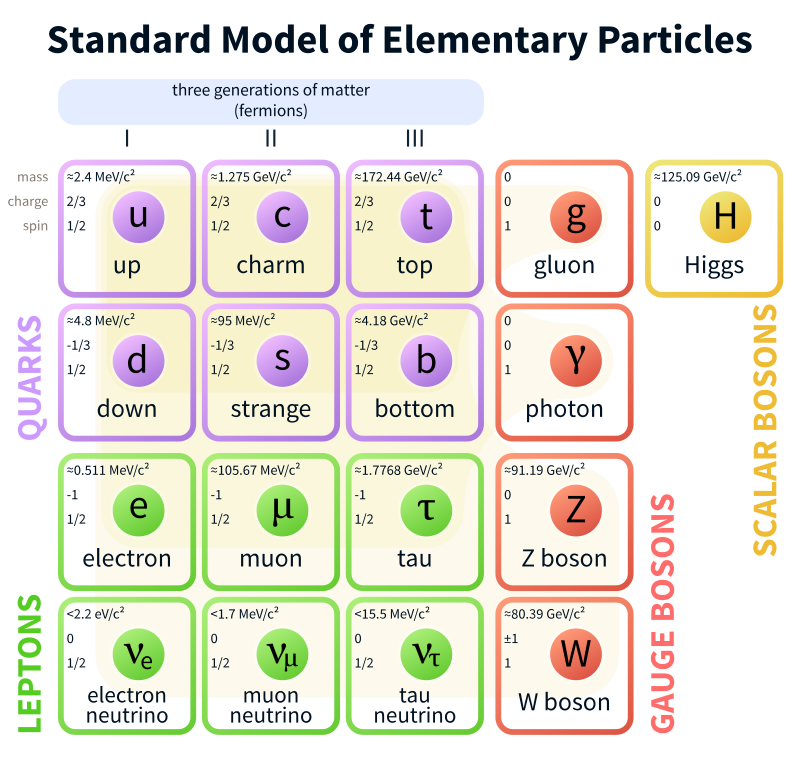 Elementary Particle is a particle whose substructure is unknown; thus, it is
unknown whether it is composed of other particles. Known elementary
particles include the fundamental fermions (quarks, leptons, antiquarks,
and antileptons), which generally are "matter particles" and "antimatter
particles", as well as the fundamental bosons (gauge bosons and the Higgs
boson), which generally are "force particles" that mediate interactions
among fermions. A particle containing two or more elementary particles is
a composite particle. (Fundamental Particle).
Elementary Particle is a particle whose substructure is unknown; thus, it is
unknown whether it is composed of other particles. Known elementary
particles include the fundamental fermions (quarks, leptons, antiquarks,
and antileptons), which generally are "matter particles" and "antimatter
particles", as well as the fundamental bosons (gauge bosons and the Higgs
boson), which generally are "force particles" that mediate interactions
among fermions. A particle containing two or more elementary particles is
a composite particle. (Fundamental Particle).Beta Particle is a high-energy, high-speed electron or positron emitted in the radioactive decay of an atomic nucleus, such as a potassium-40 nucleus, in the process of beta decay.
Causality in physics is the relationship between cause and effects. It is considered to be fundamental to all natural science, especially physics. Causality is also a topic studied from the perspectives of philosophy and statistics. Causality means that an effect cannot occur from a cause which is not in the back (past) light cone of that event. Similarly, a cause cannot have an effect outside its front (future) light cone. Why cause and effect isn’t an element of fundamental particle physics, and that’s because particles don’t care about the flow of time. For cause and effect to happen things have to occur in a linear fashion forward, “A to B.” However, the “underlying laws of physics don’t care about the direction of time,” and instead they follow predictable behaviors of a pattern. The current state of a particle doesn’t dictate its next state. Cause and effect as we think of it only exists for us in the context of time, which only moves forward.
Do Cause and Effect Really Exist? (Big Picture Ep. 2/5) (youtube) - Patterns between Events.
A system of lifeless particles can become "life-like" by collectively switching back and forth between crystalline and fluid states -- even when the environment remains stable.
Baryon is a composite subatomic particle made up of three quarks (a triquark, as distinct from mesons, which are composed of one quark and one antiquark). List of Baryons (wiki).
Lambda Baryon are a family of subatomic hadron particles containing one up quark, one down quark, and a third quark from a higher flavour generation, in a combination where the wavefunction changes sign upon the flavour of any two quarks being swapped (thus differing from a Sigma baryon). They are thus baryons, with total isospin of 0, and which are either neutral or have the elementary charge +1.
Quark is an elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. Due to a phenomenon known as color confinement, quarks are never directly observed or found in isolation; they can be found only within hadrons, such as baryons (of which protons and neutrons are examples) and mesons. For this reason, much of what is known about quarks has been drawn from observations of the hadrons themselves. Quarks have various intrinsic properties, including electric charge, mass, color charge, and spin. There are six types, known as flavors, of quarks: up, down, strange, charm, bottom, and top. Waves - Quantum.
Nucleon is one of the particles that make up the atomic nucleus. Each atomic nucleus consists of one or more nucleons, and each atom in turn consists of a cluster of nucleons surrounded by one or more electrons. There are two known kinds of nucleon: the neutron and the proton. The mass number of a given atomic isotope is identical to its number of nucleons. Thus the term nucleon number may be used in place of the more common terms mass number or atomic mass number.
Neutrino is a fermion (an elementary particle with half-integer spin) that interacts only via the weak subatomic force and gravity. The mass of the neutrino is much smaller than that of the other known elementary particles. Neutrinos typically pass through normal matter unimpeded and undetected. For each neutrino, there also exists a corresponding antiparticle, called an antineutrino, which also has half-integer spin and no electric charge. They are distinguished from the neutrinos by having opposite signs of lepton number and chirality. To conserve total lepton number, in nuclear beta decay, electron neutrinos appear together with only positrons (anti-electrons) or electron-antineutrinos, and electron antineutrinos with electrons or electron neutrinos. Neutrinos are created by various radioactive decays, including in beta decay of atomic nuclei or hadrons, nuclear reactions such as those that take place in the core of a star or artificially in nuclear reactors, nuclear bombs or particle accelerators, during a supernova, in the spin-down of a neutron star, or when accelerated particle beams or cosmic rays strike atoms. The majority of neutrinos in the vicinity of the Earth are from nuclear reactions in the Sun. In the vicinity of the Earth, about 65 billion (6.5×1010) solar neutrinos per second pass through every square centimeter perpendicular to the direction of the Sun. Neutrino Detector is a physics apparatus which is designed to study neutrinos. Because neutrinos only weakly interact with other particles of matter, neutrino detectors must be very large to detect a significant number of neutrinos. Neutrino detectors are often built underground, to isolate the detector from cosmic rays and other background radiation. Electron neutrinos are produced in the Sun as a product of nuclear fusion. Solar neutrinos constitute by far the largest flux of neutrinos from natural sources observed on Earth, as compared with e.g. atmospheric neutrinos or the diffuse supernova neutrino background. Neutrino Oscillation is a quantum mechanical phenomenon whereby a neutrino created with a specific lepton family number ("lepton flavor": electron, muon, or tau) can later be measured to have a different lepton family number. The probability of measuring a particular flavor for a neutrino varies between 3 known states, as it propagates through space. Neutralino (wiki).
IceCube Neutrino Observatory - Wiki - Antarctic Muon And Neutrino Detector Array (wiki)
Anti-Neutrinos are produced in nuclear beta decay together with a beta particle, in which, e.g., a neutron decays into a proton, electron, and antineutrino. Differences in the behavior of Neutrinos and Antineutrinos. Neutrinos are fundamental particles but do not interact with normal matter very strongly, such that around 50 trillion neutrinos from the Sun pass through your body every second. Antimatter.
Geoneutrino is an electron antineutrino emitted in β−decay of a radionuclide naturally occurring in the Earth. Neutrinos, the lightest of the known subatomic particles, lack measurable electromagnetic properties and interact only via the weak nuclear force when ignoring gravity.
Fermion is a particle that follows Fermi–Dirac statistics. These particles obey the Pauli exclusion principle. Fermions include all quarks and leptons, as well as all composite particles made of an odd number of these, such as all baryons and many atoms and nuclei. Fermions differ from bosons, which obey Bose–Einstein statistics. Majorana Fermion is a fermion that is its own antiparticle.
Lepton is an elementary, half-integer spin (spin 1⁄2) particle that does not undergo strong interactions. Two main classes of leptons exist: charged leptons (also known as the electron-like leptons), and neutral leptons (better known as neutrinos). Charged leptons can combine with other particles to form various composite particles such as atoms and positronium, while neutrinos rarely interact with anything, and are consequently rarely observed. The best known of all leptons is the electron.
Gluino is the hypothetical supersymmetric partner of a gluon. Should they exist, gluinos are expected by supersymmetry theorists to be pair produced in particle accelerators such as the Large Hadron Collider.
Quark Gluon Plasma is a state of matter in quantum chromodynamics (QCD) which exists at extremely high temperature and/or density. This state is thought to consist of asymptotically free quarks and gluons, which are several of the basic building blocks of matter. It is believed that up to a few milliseconds after the Big Bang, known as the Quark epoch, the Universe was in a quark–gluon plasma state. In June 2015, an international team of physicists produced quark-gluon plasma at the Large Hadron Collider by colliding protons with lead nuclei at high energy inside the supercollider’s Compact Muon Solenoid detector. They also discovered that this newly produced state of matter behaves like a fluid.
Gluon are elementary particles that act as the exchange particles (or gauge bosons) for the strong force between quarks, analogous to the exchange of photons in the electromagnetic force between two charged particles. In lay terms, they "glue" quarks together, forming protons and neutrons.
Muon is an elementary particle similar to the electron, with an electric charge of −1 e and a spin of 1/2, but with a much greater mass. It is classified as a lepton. As is the case with other leptons, the muon is not believed to have any sub-structure—that is, it is not thought to be composed of any simpler particles.
Dibaryon are a large family of hypothetical particles, each particle consisting of six quarks or antiquarks of any flavours. Six constituent quarks in any of several combinations could yield a colour charge of zero; for example a hexaquark might contain either six quarks, resembling two baryons bound together (a dibaryon), or three quarks and three antiquarks. Once formed, dibaryons are predicted to be fairly stable by the standards of particle physics. In 1977 Robert Jaffe proposed that a possibly stable H dibaryon with the quark composition udsuds could notionally result from the combination of two uds hyperons.
Flavour refers to a species of an elementary particle. The Standard Model counts six flavours of quarks and six flavours of leptons. They are conventionally parameterized with flavour quantum numbers that are assigned to all subatomic particles, including composite ones. For hadrons, these quantum numbers depend on the numbers of constituent quarks of each particular flavour.
Strangelet is a hypothetical particle consisting of a bound state of roughly equal numbers of up, down, and strange quarks. An equivalent description is that a strangelet is a small fragment of strange matter, small enough to be considered a particle. The size of an object composed of strange matter could, theoretically, range from a few femtometers across (with the mass of a light nucleus) to arbitrarily large. Once the size becomes macroscopic (on the order of metres across), such an object is usually called a strange star. The term "strangelet" originates with Edward Farhi and R. L. Jaffe. Strangelets have been suggested as a dark matter candidate.
Photino is a hypothetical subatomic particle, the fermion WIMP superpartner of the photon predicted by supersymmetry. It is an example of a gaugino. Even though no photino has ever been observed so far, it is expected to be the lightest stable particle in the universe. It is proposed that photinos are produced by sources of ultra-high-energy cosmic rays.
String Theory is a theoretical framework in which the point-like particles of particle physics are replaced by one-dimensional objects called strings. It describes how these strings propagate through space and interact with each other. On distance scales larger than the string scale, a string looks just like an ordinary particle, with its mass, charge, and other properties determined by the vibrational state of the string. In string theory, one of the many vibrational states of the string corresponds to the graviton, a quantum mechanical particle that carries gravitational force. Thus string theory is a theory of quantum gravity.
Superstring theory is an attempt to explain all of the particles and fundamental forces of nature in one theory by modelling them as vibrations of tiny supersymmetric strings.
Axion is a hypothetical elementary particle postulated by the Peccei–Quinn theory in 1977 to resolve the strong CP problem in quantum chromodynamics (QCD). If axions exist and have low mass within a specific range, they are of interest as a possible component of cold dark matter.
M-theory is a theory in physics that unifies all consistent versions of superstring theory. The existence of such a theory was first conjectured by Edward Witten at a string theory conference at the University of Southern California in the spring of 1995. Witten's announcement initiated a flurry of research activity known as the second superstring revolution.
Introduction to M-theory presents an idea about the basic substance of the universe. So far no experimental evidence exists showing that M-theory is a description of the real world. Interest in this theory is mainly driven by mathematical elegance.
Symmetry (math) - Balance - Quantum
Dimensions - Shapes - Geometry
Strong CP Problem is a puzzling question in particle physics: Why does quantum chromodynamics (QCD) seem to preserve CP-symmetry? In particle physics, CP stands for Charge+Parity or Charge-conjugation Parity symmetry: the combination of charge conjugation symmetry (C) and parity symmetry (P). According to the current mathematical formulation of quantum chromodynamics, a violation of CP-symmetry in strong interactions could occur. However, no violation of the CP-symmetry has ever been seen in any experiment involving only the strong interaction. As there is no known reason in QCD for it to necessarily be conserved, this is a "fine tuning" problem known as the strong CP problem. The strong CP problem is sometimes regarded as an unsolved problem in physics, and has been referred to as "the most underrated puzzle in all of physics." There are several proposed solutions to solve the strong CP problem. The most well-known is Peccei–Quinn theory, involving new pseudoscalar particles called axions.
Charge Parity is a multiplicative quantum number of some particles that describes their behavior under the symmetry operation of charge conjugation. Charge conjugation changes the sign of all quantum charges (that is, additive quantum numbers), including the electrical charge, baryon number and lepton number, and the flavor charges strangeness, charm, bottomness, topness and Isospin (I3). In contrast, it doesn't affect the mass, linear momentum or spin of a particle.
C-Symmetry is a transformation that switches all particles with their corresponding antiparticles, and thus changes the sign of all charges: not only electric charge but also the charges relevant to other forces. In physics, C-symmetry means the symmetry of physical laws under a charge-conjugation transformation. Electromagnetism, gravity and the strong interaction all obey C-symmetry, but weak interactions violate C-symmetry.
CPT Symmetry is a fundamental symmetry of physical laws under the simultaneous transformations of charge conjugation (C), parity transformation (P), and time reversal (T). CPT is the only combination of C, P, and T that is observed to be an exact symmetry of nature at the fundamental level.
Spatial Intelligence - Holography (virtual reality)
Cosmological Constant is the value of the energy density of the vacuum of space.
Theory of Everything is a hypothetical single, all-encompassing, coherent theoretical framework of physics that fully explains and links together all physical aspects of the universe. Finding a ToE is one of the major unsolved problems in physics.
Relativity Info-Graph (image)
Alternatives to General Relativity are physical theories that attempt to describe the phenomena of gravitation in competition to Einstein's theory of general relativity.
Electromagnetism - Fields
Graviton is a hypothetical elementary particle that mediates the force of gravitation in the framework of quantum field theory.
Interference Wave Propagation is a phenomenon in which two waves superpose to form a resultant wave of greater, lower, or the same amplitude. Interference usually refers to the interaction of waves that are correlated or coherent with each other, either because they come from the same source or because they have the same or nearly the same frequency. Interference effects can be observed with all types of waves, for example, light, radio, acoustic, surface water waves or matter waves.
Coherence two wave sources are perfectly coherent if they have a constant phase difference and the same frequency. It is an ideal property of waves that enables stationary (i.e. temporally and spatially constant) interference. It contains several distinct concepts, which are limiting cases that never quite occur in reality but allow an understanding of the physics of waves, and has become a very important concept in quantum physics. More generally, coherence describes all properties of the correlation between physical quantities of a single wave, or between several waves or wave packets.
Dirac Equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin-1/2 massive particles such as electrons and quarks for which parity is a symmetry. It is consistent with both the principles of quantum mechanics and the theory of special relativity, and was the first theory to account fully for special relativity in the context of quantum mechanics. It was validated by accounting for the fine details of the hydrogen spectrum in a completely rigorous way.
Frequencies - Hz - Energy - Outline of Energy
Negative Energy is a concept used in physics to explain the nature of certain fields, including the gravitational field and a number of quantum field effects. In more speculative theories, negative energy is involved in wormholes which allow time travel and warp drives for faster-than-light space travel. Dark Energy - Negative Mass.
Cosmic Ray are high-energy radiation, mainly originating outside the Solar System. Upon impact with the Earth's atmosphere, cosmic rays can produce showers of secondary particles that sometimes reach the surface. Composed primarily of high-energy protons and atomic nuclei, they are of mysterious origin. Data from the Fermi space telescope (2013) have been interpreted as evidence that a significant fraction of primary cosmic rays originate from the supernovae explosions of stars. Active galactic nuclei probably also produce cosmic rays.
Ultra-High-Energy Cosmic Ray is a cosmic ray particle with a kinetic energy greater than 1×1018 eV, far beyond both the rest mass and energies typical of other cosmic ray particles. An extreme-energy cosmic ray (EECR) is an UHECR with energy exceeding 5×1019 eV (about 8 joule), the so-called Greisen–Zatsepin–Kuzmin limit (GZK limit). This limit should be the maximum energy of cosmic ray particles that have traveled long distances (about 160 million light years), since higher-energy ray particles would have lost energy over that distance due to scattering from photons in the cosmic microwave background. It follows that EECR could not be survivors from the early universe but are cosmologically "young", emitted somewhere in the Local Supercluster by some unknown physical process. These particles are extremely rare; between 2004 and 2007, the initial runs of the Pierre Auger Observatory detected 27 events with estimated arrival energies above 5.7×1019 eV, i.e., about one such event every four weeks in the 3000 km2 area surveyed by the observatory. There is evidence that these highest-energy cosmic rays might be iron nuclei, rather than the protons that make up most cosmic rays.
Electronvolt is a unit of energy equal to approximately 160 zeptojoules (10−21 joules, symbol zJ) or 1.6×10−19 joules (symbol J). By definition, it is the amount of energy gained (or lost) by the charge of a single electron moving across an electric potential difference of one volt. Thus it is 1 volt (1 joule per coulomb, 1 J/C) multiplied by the elementary charge (e, or 1.6021766208(98)×10−19 C). Therefore, one electronvolt is equal to 1.6021766208(98)×10−19 J. Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences because a particle with charge q has an energy E = qV after passing through the potential V; if q is quoted in integer units of the elementary charge and the terminal bias in volts, one gets an energy in eV.
Steric Effects arise from a fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds (Pauli or Exchange interaction, or Born repulsion), and this may affect the molecule's preferred shape (conformation) and reactivity.
Planck Length is a unit of length, equal to 1.616229(38)×10−35 metres. It is a base unit in the system of Planck units, developed by physicist Max Planck. The Planck length can be defined from three fundamental physical constants: the speed of light in a vacuum, the Planck constant, and the gravitational constant. Nano Technology.
Molecules - Space - Singularity
Alpha is an international collaboration based at CERN, and who is working with trapped antihydrogen atoms, the antimatter counterpart of the simplest atom, hydrogen. By precise comparisons of hydrogen and antihydrogen, the experiment hopes to study fundamental symmetries between matter and antimatter.
Particle Accelerator - Particle Smashing
Particle Accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams. Large accelerators are used for basic research in particle physics. The largest accelerator currently operating is the Large Hadron Collider (LHC) near Geneva, Switzerland, operated by the CERN. It is a collider accelerator, which can accelerate two beams of protons to an energy of 6.5 TeV and cause them to collide head-on, creating center-of-mass energies of 13 TeV. Other powerful accelerators are SuperKEKB at KEK in Japan, RHIC at Brookhaven National Laboratory in New York and, formerly, the Tevatron at Fermilab, Batavia, Illinois. Accelerators are also used as synchrotron light sources for the study of condensed matter physics. Smaller particle accelerators are used in a wide variety of applications, including particle therapy for oncological purposes, radioisotope production for medical diagnostics, ion implanters for manufacture of semiconductors, and accelerator mass spectrometers for measurements of rare isotopes such as radiocarbon. There are currently more than 30,000 accelerators in operation around the world. There are two basic classes of accelerators: electrostatic and electrodynamic (or electromagnetic) accelerators. Electrostatic accelerators use static electric fields to accelerate particles. The most common types are the Cockcroft–Walton generator and the Van de Graaff generator. A small-scale example of this class is the cathode ray tube in an ordinary old television set. The achievable kinetic energy for particles in these devices is determined by the accelerating voltage, which is limited by electrical breakdown. Electrodynamic or electromagnetic accelerators, on the other hand, use changing electromagnetic fields (either magnetic induction or oscillating radio frequency fields) to accelerate particles. Since in these types the particles can pass through the same accelerating field multiple times, the output energy is not limited by the strength of the accelerating field. This class, which was first developed in the 1920s, is the basis for most modern large-scale accelerators. Rolf Widerøe, Gustav Ising, Leó Szilárd, Max Steenbeck, and Ernest Lawrence are considered pioneers of this field, conceiving and building the first operational linear particle accelerator,[4] the betatron, and the cyclotron. Because the target of the particle beams of early accelerators was usually the atoms of a piece of matter, with the goal being to create collisions with their nuclei in order to investigate nuclear structure, accelerators were commonly referred to as atom smashers in the 20th century. The term persists despite the fact that many modern accelerators create collisions between two subatomic particles, rather than a particle and an atomic nucleus. Cosmic Rays.
Large Hadron Collider is the world's largest and most powerful particle collider, most complex experimental facility ever built, and the largest single machine in the world. It was built by the European Organization for Nuclear Research (CERN) between 1998 and 2008 in collaboration with over 10,000 scientists and engineers from over 100 countries, as well as hundreds of universities and laboratories. It lies in a tunnel 27 kilometres (17 mi) in circumference, as deep as 175 metres (574 ft) beneath the France–Switzerland border near Geneva, Switzerland. Its first research run took place from 30 March 2010 to 13 February 2013 at an initial energy of 3.5 teraelectronvolts (TeV) per beam (7 TeV total), almost 4 times more than the previous world record for a collider, rising to 4 TeV per beam (8 TeV total) from 2012. On 13 February 2013 the LHC's first run officially ended, and it was shut down for planned upgrades. 'Test' collisions restarted in the upgraded collider on 5 April 2015, reaching 6.5 TeV per beam on 20 May 2015 (13 TeV total, the current world record). Its second research run commenced on schedule, on 3 June 2015. The aim of the LHC is to allow physicists to test the predictions of different theories of particle physics, including measuring the properties of the Higgs boson and searching for the large family of new particles predicted by supersymmetric theories, as well as other unsolved questions of physics. The collider has four crossing points, around which are positioned seven detectors, each designed for certain kinds of research. The LHC primarily collides proton beams, but it can also use beams of lead nuclei. Proton–lead collisions were performed for short periods in 2013 and 2016, and lead–lead collisions took place in 2010, 2011, 2013, and 2015. The LHC's computing grid is a world record holder. Data from collisions was produced at an unprecedented rate for the time of first collisions, tens of petabytes per year, a major challenge at the time, to be analysed by a grid-based computer network infrastructure connecting 140 computing centres in 35 countries – by 2012 the Worldwide LHC Computing Grid was also the world's largest distributed computing grid, comprising over 170 computing facilities in a worldwide network across 36 countries. (A Sub Atomic Smash Up Derby).
Collider is a type of particle accelerator involving directed beams of particles. Colliders may either be ring accelerators or linear accelerators, and may collide a single beam of particles against a stationary target or two beams head-on. Colliders are used as a research tool in particle physics by accelerating particles to very high kinetic energy and letting them impact other particles. Analysis of the byproducts of these collisions gives scientists good evidence of the structure of the subatomic world and the laws of nature governing it. These may become apparent only at high energies and for tiny periods of time, and therefore may be hard or impossible to study in other ways. Ray Tubes.
Step inside the Large Hadron Collider (360 video) - BBC News (youtube)
Boson is a particle that follows Bose–Einstein statistics. Bosons make up one of the two classes of particles, the other being fermions. The name boson was coined by Paul Dirac to commemorate the contribution of the Indian physicist Satyendra Nath Bose in developing, with Einstein, Bose–Einstein statistics—which theorizes the characteristics of elementary particles. Examples of bosons include fundamental particles such as photons, gluons, and W and Z bosons (the four force-carrying gauge bosons of the Standard Model), the recently discovered Higgs boson, and the hypothetical graviton of quantum gravity; composite particles (e.g. mesons and stable nuclei of even mass number such as deuterium (with one proton and one neutron, mass number = 2), helium-4, or lead-208); and some quasiparticles (e.g. Cooper pairs, plasmons, and phonons).
Higgs Boson is an elementary particle in the Standard Model of particle physics. It is the quantum excitation of the Higgs field, a fundamental field of crucial importance to particle physics theory first suspected to exist in the 1960s. Unlike other known fields such as the electromagnetic field, it has a non-zero constant value in vacuum. The question of the Higgs field's existence has been the last unverified part of the Standard Model of particle physics and, according to some, "the central problem in particle physics. Higgs Boson T-Shirts.
Bose–Einstein Condensate (BEC) is a state of matter of a dilute gas of bosons cooled to temperatures very close to absolute zero. Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which point microscopic quantum phenomena, particularly wavefunction interference, become apparent. A BEC is formed by cooling a gas of extremely low density, about one-hundred-thousandth the density of normal air, to ultra-low temperatures. This state was first predicted, generally, in 1924–25 by Satyendra Nath Bose and Albert Einstein.
Tevatron was a circular particle accelerator (now inactive, since 2011) in the United States, at the Fermi National Accelerator Laboratory (also known as Fermilab), just east of Batavia, Illinois, and holds the title of the second highest energy particle collider in the world, after the Large Hadron Collider (LHC) of the European Organization for Nuclear Research (CERN) near Geneva, Switzerland. The Tevatron was a synchrotron that accelerated protons and antiprotons in a 6.86 km, or 4.26 mi, ring to energies of up to 1 TeV, hence its name. The Tevatron was completed in 1983 at a cost of $120 million and significant upgrade investments were made in 1983–2011.
Fusion - Biology - Telescopes - Science Kits - Science Tools - Computers - Electricity
Synchrotron is a particular type of cyclic particle accelerator, descended from the cyclotron, in which the accelerating particle beam travels around a fixed closed-loop path. The magnetic field which bends the particle beam into its closed path increases with time during the accelerating process, being synchronized to the increasing kinetic energy of the particles . The synchrotron is one of the first accelerator concepts to enable the construction of large-scale facilities, since bending, beam focusing and acceleration can be separated into different components. The most powerful modern particle accelerators use versions of the synchrotron design. The largest synchrotron-type accelerator is the 27-kilometre-circumference (17 mi) Large Hadron Collider (LHC) near Geneva, Switzerland, built in 2008 by the European Organization for Nuclear Research (CERN).
Quantum Physics
Quantum Physics is a fundamental branch of physics concerned with processes involving, for example, atoms and photons. Systems such as these which obey quantum mechanics can be in a quantum superposition of different states, unlike in classical physics. Action Physics.
Quantum is the minimum amount of any physical entity involved in an interaction. The fundamental notion that a physical property may be "quantized" is referred to as "the hypothesis of quantization". This means that the magnitude of the physical property can take on only certain discrete values. Quantum is a discrete amount of something that is analogous to the quantities in quantum theory.
Particles - Nano Technologies - Quantum Computing.
Quanta is the smallest discrete quantity of some physical property that a system can possess (according to quantum theory).
Up Quark is the lightest of all quarks, a type of elementary particle, and a major constituent of matter. It, along with the down quark, forms the neutrons (one up quark, two down quarks) and protons (two up quarks, one down quark) of atomic nuclei.
Down Quark is the second-lightest of all quarks, a type of elementary particle, and a major constituent of matter. Together with the up quark, it forms the neutrons (one up quark, two down quarks) and protons (two up quarks, one down quark) of atomic nuclei.
Quantum Superposition states that, much like waves in classical physics, any two (or more) quantum states can be added together ("superposed") and the result will be another valid quantum state; and conversely, that every quantum state can be represented as a sum of two or more other distinct states. Mathematically, it refers to a property of solutions to the Schrödinger equation; since the Schrödinger equation is linear, any linear combination of solutions will also be a solution. An example of a physically observable manifestation of the wave nature of quantum systems is the interference peaks from an electron beam in a double-slit experiment. The pattern is very similar to the one obtained by diffraction of classical waves. Another example is a quantum logical qubit state, as used in quantum information processing, which is a quantum superposition of the "basis states". Quantum Super Position is a fundamental principle of quantum mechanics.
Superposition Principle states that, for all linear systems, the net response at a given place and time caused by two or more stimuli is the sum of the responses that would have been caused by each stimulus individually. So that if input A produces response X and input B produces response Y then input (A + B) produces response (X + Y). Quantum Computers.
Quantum Zeno Effect is a situation in which an unstable particle, if observed continuously, will never decay. One can "freeze" the evolution of the system by measuring it frequently enough in its known initial state.
Quantum Entanglement is a physical phenomenon that occurs when pairs or groups of particles are generated or interact in ways such that the quantum state of each particle cannot be described independently of the others, even when the particles are separated by a large distance—instead, a quantum state must be described for the system as a whole.
There are six types or flavors of quarks: up, down, strange, charm, bottom, and top. Up and down quarks have the lowest masses of all quarks. The heavier quarks rapidly change into up and down quarks through a process of particle decay: the transformation from a higher mass state to a lower mass state. Quantum Entanglement.
Pauli Exclusion Principle is the quantum mechanical principle which states that two or more identical fermions (particles with half-integer spin) cannot occupy the same quantum state within a quantum system simultaneously. In the case of electrons in atoms, it can be stated as follows: it is impossible for two electrons of a poly-electron atom to have the same values of the four quantum numbers. (n=1, L=0, M=0 S=minus half) (n=1, L=0, M=0 S=plus half).
Analogous is similar or equivalent in some respects though otherwise dissimilar. Corresponding in function but not in evolutionary origin.
Involving is to connect closely and often incriminatingly. Engage as a participant. Contain as a part. Have as a necessary feature. Make complex or intricate or complicated.
Quantum Error Correction is used in quantum computing to protect quantum information from errors due to decoherence and other quantum noise. Quantum error correction is essential if one is to achieve fault-tolerant quantum computation that can deal not only with noise on stored quantum information, but also with faulty quantum gates, faulty quantum preparation, and faulty measurements.
Spontaneous Quantum Error Correction Demonstrated. New research tackles a central challenge of powerful quantum computing. Physicists take a step toward building a fault-tolerant quantum computer. They have realized a novel type of QEC where the quantum errors are spontaneously corrected.
Quantum Mechanics is a fundamental branch of physics concerned with processes involving, for example, atoms and photons. Systems such as these which obey quantum mechanics can be in a quantum superposition of different states, unlike in classical physics. Quantum mechanics is also known as quantum physics or quantum theory or quantum field theory. List of Equations in Quantum Mechanics (wiki).
Interpretations of quantum mechanics is a set of statements which attempt to explain how quantum mechanics informs our understanding of nature.
Quantum Theory is a physical theory that certain properties occur only in discrete amounts. Constituting a separate entity or part. Easy Explanation of Quantum Theory - Documentary (youtube).
Bell's Theorem proves that quantum physics is incompatible with local hidden variable theories. It was introduced by physicist John Stewart Bell in a 1964 paper titled "On the Einstein Podolsky Rosen Paradox", referring to a 1935 thought experiment that Albert Einstein, Boris Podolsky and Nathan Rosen used to argue that quantum physics is an "incomplete" theory. By 1935, it was already recognized that the predictions of quantum physics are probabilistic. Einstein, Podolsky and Rosen presented a scenario that, in their view, indicated that quantum particles, like electrons and photons, must carry physical properties or attributes not included in quantum theory, and the uncertainties in quantum theory's predictions are due to ignorance of these properties, later termed "hidden variables". Their scenario involves a pair of widely separated physical objects, prepared in such a way that the quantum state of the pair is entangled.
Renormalization is a collection of techniques in quantum field theory, the statistical mechanics of fields, and the theory of self-similar geometric structures, that are used to treat infinities arising in calculated quantities by altering values of quantities to compensate for effects of their self-interactions.
Quantum Levitation (youtube) - Quantum Gravity
Quantum Tunneling refers to the quantum mechanical phenomenon where a particle tunnels through a barrier that it classically could not surmount. Fusion.
Proton Tunneling is a type of quantum tunneling involving the instantaneous disappearance of a proton in one site and the appearance of the same proton at an adjacent site separated by a potential barrier. The two available sites are bounded by a double well potential of which its shape, width and height are determined by a set of boundary conditions. According to the WKB approximation, the probability for a particle to tunnel is inversely proportional to its mass and the width of the potential barrier. Electron Tunneling is well-known. A proton is about 2000 times more massive than an electron, so it has a much lower probability of tunneling; nevertheless, proton tunneling still occurs especially at low temperatures and high pressures where the width of the potential barrier is decreased. Observation Flaws.
Grotthuss Mechanism is the process by which an 'excess' proton or proton defect diffuses through the hydrogen bond network of water molecules or other hydrogen-bonded liquids through the formation and concomitant cleavage of covalent bonds involving neighboring molecules.
Quantum Decoherence is the loss of quantum coherence.
Quantum Biology refers to applications of quantum mechanics and theoretical chemistry to biological objects and problems.
Quantum Electrodynamics is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.
Quantum Chromodynamics is the theory of the strong interaction between quarks and gluons, the fundamental particles that make up composite hadrons such as the proton, neutron and pion. QCD is a type of quantum field theory called a non-abelian gauge theory, with symmetry group SU(3). The QCD analog of electric charge is a property called color. Gluons are the force carrier of the theory, like photons are for the electromagnetic force in quantum electrodynamics. The theory is an important part of the Standard Model of particle physics. A large body of experimental evidence for QCD has been gathered over the years. QCD: The Strongest Force in the Universe Visualized: Quantum Chromodynamics (youtube).
Color Charge is a property of quarks and gluons that is related to the particles' strong interactions in the theory of quantum chromodynamics.
 Evidence for a New Property of Quantum Matter Revealed. Electrical
dipole activity detected in a quantum material unlike any other tested.
The material, first synthesized 20 years ago, is called k-(BEDT-TTF)2Hg(SCN)2
Br. It is derived from organic compounds, but behaves like a metal.
Evidence for a New Property of Quantum Matter Revealed. Electrical
dipole activity detected in a quantum material unlike any other tested.
The material, first synthesized 20 years ago, is called k-(BEDT-TTF)2Hg(SCN)2
Br. It is derived from organic compounds, but behaves like a metal. Layered Double Hydroxides are a class of ionic solids characterized by a layered structure with the generic layer sequence [AcBZAcB]n, where c represents layers of metal cations, A and B are layers of hydroxide (HO−) anions, and Z are layers of other anions and neutral molecules (such as water). Lateral offsets between the layers may result in longer repeating periods. The intercalated anions (Z) are weakly bound, often exchangeable; their intercalation properties have scientific and commercial interest. LDHs occur in nature as minerals, as byproducts of metabolism of certain bacteria, and also unintentionally in man-made contexts, such as the products of corrosion of metal objects.
Statistical Mechanics describes how macroscopic observations (such as temperature and pressure) are related to microscopic parameters that fluctuate around an average. It connects thermodynamic quantities (such as heat capacity) to microscopic behavior, whereas, in classical thermodynamics, the only available option would be to measure and tabulate such quantities for various materials. Statistical mechanics is necessary for the fundamental study of any physical system that has many degrees of freedom. The approach is based on statistical methods, probability theory and the microscopic physical laws. It can be used to explain the thermodynamic behaviour of large systems. This branch of statistical mechanics, which treats and extends classical thermodynamics, is known as statistical thermodynamics or equilibrium statistical mechanics. Statistical mechanics can also be used to study systems that are out of equilibrium. An important sub-branch known as non-equilibrium statistical mechanics (sometimes called statistical dynamics) deals with the issue of microscopically modelling the speed of irreversible processes that are driven by imbalances. Examples of such processes include chemical reactions or flows of particles and heat. The fluctuation–dissipation theorem is the basic knowledge obtained from applying non-equilibrium statistical mechanics to study the simplest non-equilibrium situation of a steady state current flow in a system of many particles.
Relativistic Mechanics refers to mechanics compatible with special relativity (SR) and general relativity (GR). It provides a non-quantum mechanical description of a system of particles, or of a fluid, in cases where the velocities of moving objects are comparable to the speed of light c. As a result, classical mechanics is extended correctly to particles traveling at high velocities and energies, and provides a consistent inclusion of electromagnetism with the mechanics of particles.
Classical Mechanics is a physical theory describing the motion of macroscopic objects, from projectiles to parts of machinery, and astronomical objects, such as spacecraft, planets, stars and galaxies. For objects governed by classical mechanics, if the present state is known, it is possible to predict how it will move in the future (determinism) and how it has moved in the past (reversibility). Classical Mechanics is one of the two major sub-fields of mechanics, along with quantum mechanics. Classical mechanics is concerned with the set of physical laws describing the motion of bodies under the influence of a system of forces. The study of the motion of bodies is an ancient one, making classical mechanics one of the oldest and largest subjects in science, engineering and technology. It is also widely known as Newtonian mechanics.
Mechanics is the area of physics concerned with the motions of physical objects. Forces applied to objects result in displacements, or changes of an object's position relative to its environment.
Hamiltonian in quantum mechanics, the Hamiltonian of a system is an operator corresponding to the total energy of that system, including both kinetic energy and potential energy. Its spectrum, the system's energy spectrum or its set of energy eigenvalues, is the set of possible outcomes obtainable from a measurement of the system's total energy. Due to its close relation to the energy spectrum and time-evolution of a system, it is of fundamental importance in most formulations of quantum theory. Hamiltonian Mechanics is a mathematically sophisticated formulation of classical mechanics.
Correspondence Principle states that the behavior of systems described by the theory of quantum mechanics (or by the old quantum theory) reproduces classical physics in the limit of large quantum numbers. In other words, it says that for large orbits and for large energies, quantum calculations must agree with classical calculations.
Copenhagen Interpretation
Quantum Dot are tiny semiconductor particles a few nanometres in size, having optical and electronic properties that differ from larger particles due to quantum mechanics. They are a central topic in nanotechnology. When the quantum dots are illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy by the emission of light. This light emission (photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band.
Modified Quantum Dots capture more energy from light and lose less to heat. Los Alamos National Laboratory scientists have synthesized magnetically-doped quantum dots that capture the kinetic energy of electrons created by ultraviolet light before it's wasted as heat. Office Science.
Artificial Atoms create stable qubits for quantum computing. Quantum engineers from UNSW Sydney have created artificial atoms in silicon chips that offer improved stability for quantum computing. UNSW quantum computing researchers describe how they created artificial atoms in a silicon 'quantum dot', a tiny space in a quantum circuit where electrons are used as qubits (or quantum bits), the basic units of quantum information. Scientia Professor Andrew Dzurak explains that unlike a real atom, an artificial atom has no nucleus, but it still has shells of electrons whizzing around the centre of the device, rather than around the atom's nucleus. Artificial Relativistic Molecules.
The spin state story: Observation of the quantum spin liquid state in novel material. New insight into the spin behavior in an exotic state of matter puts us closer to next-generation spintronic devices. The quantum spin liquid (QSL) state is an exotic state of matter where the spin of electrons, which generally exhibits order at low temperatures, remains disordered. Now, scientists have developed a new material where a two-dimensional QSL state can be experimentally observed, advancing our knowledge of spin behavior, and getting us closer to next-generation ''spintronic'' devices.
Fields
 Quantum Field
Theory is the theoretical framework for constructing quantum
mechanical models of subatomic particles in particle physics and
quasiparticles in condensed matter physics. QFT treats particles as
excited states of the underlying physical field, so these are called field
quanta. Quantum Field Theory is a theoretical framework that combines
classical field theory, special relativity, and quantum mechanics:xi (but
notably not general relativity's description of gravity) and is used to
construct physical models of subatomic particles (in particle physics) and
quasiparticles (in condensed matter physics). QFT treats particles as
excited states (also called quanta) of their underlying fields, which are
more fundamental than the particles. Interactions between particles are
described by interaction terms in the Lagrangian involving their
corresponding fields. Each interaction can be visually represented by
Feynman diagrams, which are formal computational tools, in the process of
relativistic perturbation theory.
Quantum Field
Theory is the theoretical framework for constructing quantum
mechanical models of subatomic particles in particle physics and
quasiparticles in condensed matter physics. QFT treats particles as
excited states of the underlying physical field, so these are called field
quanta. Quantum Field Theory is a theoretical framework that combines
classical field theory, special relativity, and quantum mechanics:xi (but
notably not general relativity's description of gravity) and is used to
construct physical models of subatomic particles (in particle physics) and
quasiparticles (in condensed matter physics). QFT treats particles as
excited states (also called quanta) of their underlying fields, which are
more fundamental than the particles. Interactions between particles are
described by interaction terms in the Lagrangian involving their
corresponding fields. Each interaction can be visually represented by
Feynman diagrams, which are formal computational tools, in the process of
relativistic perturbation theory. Classical Field Theory is a physical theory that predicts how one or more physical fields interact with matter through field equations. The term 'classical field theory' is commonly reserved for describing those physical theories that describe electromagnetism and gravitation, two of the fundamental forces of nature. Theories that incorporate quantum mechanics are called quantum field theories. Waves.
Unified Field Theory is a type of field theory that allows all that is usually thought of as fundamental forces and elementary particles to be written in terms of a single field.
Field Equation is a partial differential equation which determines the dynamics of a physical field, specifically the time evolution and spatial distribution of the field. The solutions to the equation are mathematical functions which correspond directly to the field, as a functions of time and space. Since the field equation is a partial differential equation, there are families of solutions which represent a variety of physical possibilities. Usually, there is not just a single equation, but a set of coupled equations which must be solved simultaneously. Field equations are not ordinary differential equations since a field depends on space and time, which requires at least two variables.
Field is a physical quantity, represented by a number or tensor, that has a value for each point in space-time. For example, on a weather map, the surface temperature is described by assigning a real number to each point on a map; the temperature can be considered at a fixed point in time or over some time interval, to study the dynamics of temperature change. A surface wind map, assigning a vector to each point on a map that describes the wind velocity at that point, would be an example of a 1-dimensional tensor field, i.e. a vector field. Field theories, mathematical descriptions of how field values change in space and time, are ubiquitous in physics. For instance, the electric field is another rank-1 tensor field, and the full description of electrodynamics can be formulated in terms of two interacting vector fields at each point in space-time, or as a single-rank 2-tensor field theory.
Vector Field is an assignment of a vector to each point in a subset of space. A vector field in the plane (for instance), can be visualised as a collection of arrows with a given magnitude and direction, each attached to a point in the plane. Vector fields are often used to model, for example, the speed and direction of a moving fluid throughout space, or the strength and direction of some force, such as the magnetic or gravitational force, as it changes from one point to another point.
 Electric Field surrounds an electric charge,
and exerts force on other charges in the field, attracting or repelling
them. Electric field is sometimes abbreviated as E-field. The electric
field is defined mathematically as a vector field that associates to each
point in space the (electrostatic or
Coulomb) force per unit of charge exerted on an infinitesimal positive
test charge at rest at that point. The SI unit for electric field strength
is volt per meter (V/m). Newtons per coulomb (N/C) is also used as a unit
of electric field strength. Electric fields are created by electric
charges, or by time-varying magnetic fields. Electric fields are important
in many areas of physics, and are exploited practically in electrical
technology. On an atomic scale, the electric field is responsible for the
attractive force between the atomic nucleus and
electrons that holds atoms together, and the
forces between atoms that cause
chemical bonding. Electric
fields and magnetic fields are both manifestations of the
electromagnetic force, one of the four
fundamental forces (or interactions) of nature.
Electric Motors.
Electric Field surrounds an electric charge,
and exerts force on other charges in the field, attracting or repelling
them. Electric field is sometimes abbreviated as E-field. The electric
field is defined mathematically as a vector field that associates to each
point in space the (electrostatic or
Coulomb) force per unit of charge exerted on an infinitesimal positive
test charge at rest at that point. The SI unit for electric field strength
is volt per meter (V/m). Newtons per coulomb (N/C) is also used as a unit
of electric field strength. Electric fields are created by electric
charges, or by time-varying magnetic fields. Electric fields are important
in many areas of physics, and are exploited practically in electrical
technology. On an atomic scale, the electric field is responsible for the
attractive force between the atomic nucleus and
electrons that holds atoms together, and the
forces between atoms that cause
chemical bonding. Electric
fields and magnetic fields are both manifestations of the
electromagnetic force, one of the four
fundamental forces (or interactions) of nature.
Electric Motors.Magnetic Field is a vector field that describes the magnetic influence of electric charges in relative motion and magnetized materials. The effects of magnetic fields are commonly seen in permanent magnets, which pull on magnetic materials (such as iron) and attract or repel other magnets. Magnetic fields surround and are created by magnetized material and by moving electric charges (electric currents) such as those used in electromagnets. They exert forces on nearby moving electrical charges and torques on nearby magnets. In addition, a magnetic field that varies with location exerts a force on magnetic materials. Both the strength and direction of a magnetic field vary with location. As such, it is described mathematically as a vector field.
Hamiltonian Field Theory is the field-theoretic analogue to classical Hamiltonian mechanics. It is a formalism in classical field theory alongside Lagrangian field theory. It also has applications in quantum field theory. Waves.
Quantum Entanglement (connectedness) - Quantum Gravity (space) - Quantum Computing (super computers) - Time - Thermodynamics.
Scalar Field associates a scalar value to every point in a space – possibly physical space. The scalar may either be a (dimensionless) mathematical number or a physical quantity. In a physical context, scalar fields are required to be independent of the choice of reference frame, meaning that any two observers using the same units will agree on the value of the scalar field at the same absolute point in space (or spacetime) regardless of their respective points of origin. Examples used in physics include the temperature distribution throughout space, the pressure distribution in a fluid, and spin-zero quantum fields, such as the Higgs field. These fields are the subject of scalar field theory. Scalar in mathematics is an element of a field which is used to define a vector space. A quantity described by multiple scalars, such as having both direction and magnitude, is called a vector.
Force Field is a barrier made of energy, plasma, or particles. It protects a person, area, or object from attacks or intrusions. This fictional technology is created as a field of energy without mass that acts as a wall, so that objects affected by the particular force relating to the field are unable to pass through the field and reach the other side. This concept has become a staple of many science-fiction works, so much that authors frequently do not even bother to explain or justify them to their readers, treating them almost as established fact and attributing whatever capabilities the plot requires. Force Field is sometimes known as an energy shield, force shield, force bubble, defence shield or deflector shield.
Observations - Looking Carefully
Observation is taking a patient and careful look at something in order to record it and measure it and also discover or determine the existence or the presence of some fact or detail. Observe is the act of noticing something or paying attention to something with careful consideration. Observation is the active acquisition of information and facts that are learned from a primary source. In living beings, observation employs the senses. In science, observation can also involve the recording of data via the use of instruments. The term may also refer to any data collected during the scientific activity. Observations can be qualitative, that is, only the absence or presence of a property is noted, or quantitative if a numerical value is attached to the observed phenomenon by counting or measuring. Observation is the act of making and recording a measurement. Taking a patient look at something. A remark expressing careful consideration about something. Facts that are learned by observing and witnessing. The act of noticing or paying close attention.
Observation Flaws - Biases - Point of View - Seeing isn't always believing.
Noticing is to discover, perceive or determine the existence, presence, or fact of something. Express recognition of the presence or existence of something.
Recognize is to detect something with the senses and be fully aware or cognizant of something. Recognition is coming to understand something clearly and distinctly. The state or quality of something being acknowledged. An acceptance, as of a claim, that something is true and valid. The process of recognizing something or someone by remembering.
Regard is to look at something attentively with a long fixed look. Paying particular notice.
Identify is to establish the identity of someone or something by its distinct characteristics that are easy to perceive and can be clearly outlined.
Distinct is something not alike and different in nature or quality. Something that is easy to perceive and clearly outlined and constituting a separate entity or part.
Distinguished is something detected with the senses and marked as different. Reputation.
Scrutiny is the act of examining something closely as for mistakes with a prolonged intense look.
Evidence - Questioning - Analyzing - Inspection - Label - Symbol
Contemplate is to look at something thoughtfully and observe deep in thought. To think intently and at length and consider the possibilities. To think deeply about a subject or question over a period of time.
Relative - Knowing when you're being watched - Staring
Artifact error is any error in the perception or representation of any information, introduced by the involved equipment or techniques that were used in the observation. Calibration.
Visual Artifact are anomalies apparent during visual representation as in digital graphics and other forms of imagery, particularly microscopy. Visual artifacts can be the result of digital image processing that produces noise or distortion errors.
Glimpse is a quick look at something or a brief or incomplete view.
Slow Seeing is taking your time to look at something more carefully, either to enjoy it more or to study it more in detail.
Trace is a mark, object, or other indication of the existence of something or the passing of something. To find or discover something by investigation. A very small quantity, especially one too small to be accurately measured. Trace can also mean to copy a drawing, map, or design by drawing over its lines on a superimposed piece of transparent paper.
Naturalistic Observation (pdf) - Experiencing - Processing
Discovery as an observation is the act of detecting something new, or something "old" that had been unrecognized as meaningful. With reference to sciences and academic disciplines, discovery is the observation of new phenomena, new actions, or new events and providing new reasoning to explain the knowledge gathered through such observations with previously acquired knowledge from abstract thought and everyday experiences. A discovery may sometimes be based on earlier discoveries, collaborations, or ideas. Some discoveries represent a radical breakthrough in knowledge or technology.
OODA Loop (observe, orient, decide, and act)
Observational Study draws inferences from a sample to a population where the independent variable is not under the control of the researcher because of ethical concerns or logistical constraints. One common observational study is about the possible effect of a treatment on subjects, where the assignment of subjects into a treated group versus a control group is outside the control of the investigator. This is in contrast with experiments, such as randomized controlled trials, where each subject is randomly assigned to a treated group or a control group.
Unit of Observation is the unit described by the data that one analyzes.
Unit of Analysis is the entity that frames what is being analyzed in a study, or is the entity being studied as a whole, within which most factors of causality and change exist.
Prima Facie meaning on its first encounter or at first sight. The literal translation would be "at first face" or "at first appearance".
Empirical Law is a law induced from observation or experiment, and though valid for the particular instances observed, not to be relied on beyond the conditions on which it rests. Statistics.
What we see at that same moment in time we will sometimes be understood differently from person to person. We see the same thing but process it differently because we are using different information and different experiences to compare something to what we just saw. But even then, we will sometimes see things differently depending on what part we are focusing on. You may miss certain information because you're focusing on one part and not seeing the whole picture. Two eye witnesses are better than one eye witness, but understanding how eyesight works and how the mind works will always be the key factor to understanding.
Monitoring is the act of observing something (and sometimes keeping a record of it). Keep tabs on; keep an eye on; keep under surveillance. Check, track, or observe by means of a receiver. Environmental Monitoring.
Magnifying Small Objects
 Atomic Force Microscopy is a
very-high-resolution type of
scanning probe microscopy (SPM), with demonstrated
resolution on the
order
of fractions of a nanometer, more than 1000 times better than the optical
diffraction limit. Scanning-force Microscopy (SFM).
Atomic Force Microscopy is a
very-high-resolution type of
scanning probe microscopy (SPM), with demonstrated
resolution on the
order
of fractions of a nanometer, more than 1000 times better than the optical
diffraction limit. Scanning-force Microscopy (SFM).Scanning Probe Microscopy is a branch of microscopy that forms images of surfaces using a physical probe that scans the specimen.
Gamma Spectroscopy identify atoms by detecting the energy of gamma rays.
Crookes Tube is an early experimental electrical discharge tube, with vacuum, used to discover the properties of cathode rays.
Electron Microscope is a microscope that uses a beam of accelerated electrons as a source of illumination.
Scanning Electron Microscope is a type of electron microscope that produces images of a sample by scanning the surface with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that contain information about the sample's surface topography and composition. The electron beam is scanned in a raster scan pattern, and the beam's position is combined with the detected signal to produce an image. SEM can achieve resolution better than 1 nanometer. Specimens can be observed in high vacuum in conventional SEM, or in low vacuum or wet conditions in variable pressure or environmental SEM, and at a wide range of cryogenic or elevated temperatures with specialized instruments.
Transmission Electron Microscopy is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a grid. An image is formed from the interaction of the electrons with the sample as the beam is transmitted through the specimen. The image is then magnified and focused onto an imaging device, such as a fluorescent screen, a layer of photographic film, or a sensor such as a charge-coupled device.
Electron Paramagnetic Resonance (EPR) is a method for studying materials with unpaired electrons.
Cryo-Electron Microscopy (cryo-EM) is a form of transmission electron microscopy (TEM) where the sample is studied at cryogenic temperatures (generally liquid-nitrogen temperatures).
Electron cryo-microscopy: Using inexpensive technology to produce high-resolution images. The trick: the samples are flash frozen and then bombarded with electrons. In the case of traditional electron microscopy, all of the water is first extracted from the sample. This is necessary because the investigation takes place in a vacuum, which means water would evaporate immediately and make imaging impossible. However, because water molecules play such an important role in biomolecules, especially in proteins, they cannot be examined using traditional electron microscopy. Proteins are among the most important building blocks of cells and perform a variety of tasks. In-depth knowledge of their structure is necessary in order to understand how they work.
Scanning Tunneling Microscope is an instrument for imaging surfaces at the atomic level.
Sizes (nano) - Telescopes (lens) - Sensors (chromatography)
Ghost imaging speeds up super-resolution microscopy. New nanoscopy approach poised to capture biological processes occurring inside cells at submillisecond speeds.
Lattice-Light-Sheet Microscopy - Video (vimeo)
Light Sheet Fluorescence Microscopy is a fluorescence microscopy technique with an intermediate optical resolution, but good optical sectioning capabilities and high speed.
Optical Coherence Tomography is an imaging technique that uses low-coherence light to capture micrometer-resolution, two- and three-dimensional images from within optical scattering media (e.g., biological tissue). It is used for medical imaging and industrial nondestructive testing or NDT. Optical coherence tomography is based on low-coherence interferometry, typically employing near-infrared light. The use of relatively long wavelength light allows it to penetrate into the scattering medium. Confocal microscopy, another optical technique, typically penetrates less deeply into the sample but with higher resolution. Depending on the properties of the light source (superluminescent diodes, ultrashort pulsed lasers, and supercontinuum lasers have been employed), optical coherence tomography has achieved sub-micrometer resolution (with very wide-spectrum sources emitting over a ~100 nm wavelength range). Optical coherence tomography is one of a class of optical tomographic techniques. Commercially available optical coherence tomography systems are employed in diverse applications, including art conservation and diagnostic medicine, notably in ophthalmology and optometry where it can be used to obtain detailed images from within the retina. Recently, it has also begun to be used in interventional cardiology to help diagnose coronary artery disease, and in dermatology to improve diagnosis. A relatively recent implementation of optical coherence tomography, frequency-domain optical coherence tomography, provides advantages in the signal-to-noise ratio provided, thus permitting faster signal acquisition. Sub-surface imaging technology can expose counterfeit travel documents. New research has found that optical coherence tomography or OCT imaging technology can be utilized to distinguish between legitimate and counterfeit travel documents.
Confocal Microscopy is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a spatial pinhole to block out-of-focus light in image formation. Capturing multiple two-dimensional images at different depths in a sample enables the reconstruction of three-dimensional structures (a process known as optical sectioning) within an object. This technique is used extensively in the scientific and industrial communities and typical applications are in life sciences, semiconductor inspection and materials science. Light travels through the sample under a conventional microscope as far into the specimen as it can penetrate, while a confocal microscope only focuses a smaller beam of light at one narrow depth level at a time. The CLSM achieves a controlled and highly limited depth of focus.
Differential Interference Contrast Microscopy is an optical microscopy technique used to enhance the contrast in unstained, transparent samples. DIC works on the principle of interferometry to gain information about the optical path length of the sample, to see otherwise invisible features. A relatively complex optical system produces an image with the object appearing black to white on a grey background. This image is similar to that obtained by phase contrast microscopy but without the bright diffraction halo. Light waves travel at different speeds in different materials. Light Refraction.
Phase-Contrast Microscopy is an optical microscopy technique that converts phase shifts in light passing through a transparent specimen to brightness changes in the image. Phase shifts themselves are invisible, but become visible when shown as brightness variations. When light waves travel through a medium other than vacuum, interaction with the medium causes the wave amplitude and phase to change in a manner dependent on properties of the medium. Changes in amplitude (brightness) arise from the scattering and absorption of light, which is often wavelength-dependent and may give rise to colors. Photographic equipment and the human eye are only sensitive to amplitude variations. Without special arrangements, phase changes are therefore invisible. Yet, phase changes often carry important information. Phase-contrast microscopy is particularly important in biology. It reveals many cellular structures that are not visible with a simpler bright-field microscope, as exemplified in the figure. These structures were made visible to earlier microscopists by staining, but this required additional preparation and thus killing the cells. The phase-contrast microscope made it possible for biologists to study living cells and how they proliferate through cell division. It is one of the few methods available to quantify cellular structure and components that does not use fluorescence.
Phase Objects are samples that change the phase but not the amplitude of a light wave. In contrast, amplitude objects only affect the amplitude but not the phase of light. Flat and unstained cells almost reach the characteristics of a phase object for visible light. Polarized.
Bright-Field Microscopy is the simplest of all the optical microscopy illumination techniques. Sample illumination is transmitted (i.e., illuminated from below and observed from above) white light, and contrast in the sample is caused by attenuation of the transmitted light in dense areas of the sample. Bright-field microscopy is the simplest of a range of techniques used for illumination of samples in light microscopes, and its simplicity makes it a popular technique. The typical appearance of a bright-field microscopy image is a dark sample on a bright background, hence the name. Microbes Don’t Actually Look Like Anything (youtube) - Journey to the Microcosmos (youtube channel) .
Dark-Field Microscopy describes microscopy methods, in both light and electron microscopy, which exclude the unscattered beam from the image. As a result, the field around the specimen (i.e., where there is no specimen to scatter the beam) is generally dark.
Mirrored chip could enable handheld dark-field microscopes. Simple chip powered by quantum dots allows standard microscopes to visualize difficult-to-image biological organisms.
Mass Spectrometry is an analytical technique that ionizes chemical species and sorts the ions based on their mass-to-charge ratio. In simpler terms, a mass spectrum measures the masses within a sample. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. Atomic Spectroscopy.
Spectrometers (spectrum)
Raman Spectroscopy is a laser-based microscopic device and a spectroscopic technique used to observe vibrational, rotational, and other low-frequency modes in a system. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. The term MOLE is used to refer to the Raman-based microprobe.
Macromolecular crystallography (wiki) - Illuminating The Secrets Of Crystals-Microcrystal Electron Diffraction In Structural Biology.
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids.
Nuclear Magnetic Resonance Spectroscopy is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. Similarly, biochemists use NMR to identify proteins and other complex molecules. Besides identification, NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules. The most common types of NMR are proton and carbon-13 NMR spectroscopy, but it is applicable to any kind of sample that contains nuclei possessing spin. Nuclear Magnetic Resonance (NMR) spectroscopy is an analytical chemistry technique used in quality control and reserach for determining the content and purity of a sample as well as its molecular structure. For example, NMR can quantitatively analyze mixtures containing known compounds. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics, crystals as well as non-crystalline materials. The NMR phenomenon is based on the fact that nuclei of atoms have magnetic properties that can be utilized to yield chemical information. Quantum mechanically subatomic particles (electrons, protons and neutrons) can be imagined as spinning on their axes. (most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS)).
Nuclear Magnetic Resonance Spectroscopy of Proteins (NMR) is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and their complexes.
Nuclear Magnetic Resonance is a physical observation in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field and therefore not involving electromagnetic waves) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics, crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI). All isotopes that contain an odd number of protons and/or neutrons (see Isotope) have an intrinsic nuclear magnetic moment and angular momentum, in other words a nonzero nuclear spin, while all nuclides with even numbers of both have a total spin of zero. The most commonly used nuclei are 1H and 13C, although isotopes of many other elements can be studied by high-field NMR spectroscopy as well. A key feature of NMR is that the resonance frequency of a particular simple substance is usually directly proportional to the strength of the applied magnetic field. It is this feature that is exploited in imaging techniques; if a sample is placed in a non-uniform magnetic field then the resonance frequencies of the sample's nuclei depend on where in the field they are located. Since the resolution of the imaging technique depends on the magnitude of the magnetic field gradient, many efforts are made to develop increased gradient field strength. The principle of NMR usually involves three sequential steps: The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0. The perturbation of this alignment of the nuclear spins by a weak oscillating magnetic field, usually referred to as a radio-frequency (RF) pulse. The oscillation frequency required for significant perturbation is dependent upon the static magnetic field (B0) and the nuclei of observation. The detection of the NMR signal during or after the RF pulse, due to the voltage induced in a detection coil by precession of the nuclear spins around B0. After an RF pulse, precession usually occurs with the nuclei's intrinsic Larmor frequency and, in itself, does not involve transitions between spin states or energy levels. The two magnetic fields are usually chosen to be perpendicular to each other as this maximizes the NMR signal strength. The frequencies of the time-signal response by the total magnetization (M) of the nuclear spins are analyzed in NMR spectroscopy and magnetic resonance imaging. Both use applied magnetic fields (B0) of great strength, often produced by large currents in superconducting coils, in order to achieve dispersion of response frequencies and of very high homogeneity and stability in order to deliver spectral resolution, the details of which are described by chemical shifts, the Zeeman effect, and Knight shifts (in metals). The information provided by NMR can also be increased using hyperpolarization, and/or using two-dimensional, three-dimensional and higher-dimensional techniques. NMR phenomena are also utilized in low-field NMR, NMR spectroscopy and MRI in the Earth's magnetic field (referred to as Earth's field NMR), and in several types of magnetometers.
Multiangle Light Scattering describes a technique for measuring the light scattered by a sample into a plurality of angles.
Small Angle Scattering is a small-angle scattering method for structure analysis of biological materials.
New Microscope captures detailed 3-D movies of cells deep within living systems. Merging lattice light sheet microscopy with adaptive optics reveals the most detailed picture yet of subcellular dynamics in multicellular organisms.
Ultrafast Laser Spectroscopy is a spectroscopic technique that uses ultrashort pulse lasers for the study of dynamics on extremely short time scales (attoseconds to nanoseconds).
High-speed microscope illuminates biology at the speed of life. The team behind the revolutionary 3D SCAPE microscope announces today a new version of this high-speed imaging technology. They used SCAPE 2.0 to reveal previously unseen details of living creatures -- from neurons firing inside a wriggling worm to the 3D dynamics of the beating heart of a fish embryo, with far superior resolution and at speeds up to 30 times faster than their original demonstration. Time-lapse.
Dual-Polarization Interferometry is an analytical technique that probes molecular layers adsorbed to the surface of a waveguide using the evanescent wave of a laser beam. It is used to measure the conformational change in proteins, or other biomolecules, as they function (referred to as the conformation activity relationship).
Circular Dichroism is dichroism involving circularly polarized light, i.e., the differential absorption of left- and right-handed light. Left-hand circular (LHC) and right-hand circular (RHC) polarized light represent two possible spin angular momentum states for a photon, and so circular dichroism is also referred to as dichroism for spin angular momentum.
Neutron Imaging is the process of making an image with neutrons. The resulting image is based on the neutron attenuation properties of the imaged object. The resulting images have much in common with industrial X-ray images, but since the image is based on neutron attenuating properties instead of X-ray attenuation properties, some things easily visible with neutron imaging may be very challenging or impossible to see with X-Ray Imaging Techniques (and vice versa). X-rays are attenuated based on a material's density. Denser materials will stop more X-rays. With neutrons, a material's likelihood of attenuation of neutrons is not related to its density. Some light materials such as boron will absorb neutrons while hydrogen will generally scatter neutrons, and many commonly used metals allow most neutrons to pass through them. This can make neutron imaging better suited in many instances than X-ray imaging; for example, looking at O-ring position and integrity inside of metal components, such as the segments joints of a Solid Rocket Booster.
Neutron Diffraction is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of the structure of the material. The technique is similar to X-ray diffraction but due to their different scattering properties, neutrons and X-rays provide complementary information: X-Rays are suited for superficial analysis, strong x-rays from synchrotron radiation are suited for shallow depths or thin specimens, while neutrons having high penetration depth are suited for bulk samples.
X-Ray Crystallography is a technique used for determining the atomic and molecular structure of a crystal, in which the crystalline atoms cause a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their disorder, and various other information.
X-Ray Absorption Spectroscopy is a widely used technique for determining the local geometric and/or electronic structure of matter. The experiment is usually performed at synchrotron radiation facilities, which provide intense and tunable X-ray beams. Samples can be in the gas phase, solutions, or solids.
Synchrotron is a particular type of cyclic particle accelerator, descended from the cyclotron, in which the accelerating particle beam travels around a fixed closed-loop path. The magnetic field which bends the particle beam into its closed path increases with time during the accelerating process, being synchronized to the increasing kinetic energy of the particles. The synchrotron is one of the first accelerator concepts to enable the construction of large-scale facilities, since bending, beam focusing and acceleration can be separated into different components. The most powerful modern particle accelerators use versions of the synchrotron design. The largest synchrotron-type accelerator, also the largest particle accelerator in the world, is the 27-kilometre-circumference (17 mi) Large Hadron Collider (LHC) near Geneva, Switzerland, built in 2008 by the European Organization for Nuclear Research (CERN). It can accelerate beams of protons to an energy of 6.5 teraelectronvolts (TeV).
Synchrotron Radiation is the electromagnetic radiation emitted when charged particles are accelerated radially, e.g., when they are subject to an acceleration perpendicular to their velocity (a ⊥ v). It is produced, for example, in synchrotrons using bending magnets, undulators and/or wigglers. If the particle is non-relativistic, then the emission is called cyclotron emission. If, on the other hand, the particles are relativistic, sometimes referred to as ultrarelativistic, the emission is called synchrotron emission. Synchrotron radiation may be achieved artificially in synchrotrons or storage rings, or naturally by fast electrons moving through magnetic fields. The radiation produced in this way has a characteristic polarization and the frequencies generated can range over the entire electromagnetic spectrum which is also called continuum radiation.
Interferometry is a family of techniques in which waves, usually electromagnetic waves, are superimposed causing the phenomenon of interference in order to extract information. Interferometry is an important investigative technique in the fields of astronomy, fiber optics, engineering metrology, optical metrology, oceanography, seismology, spectroscopy (and its applications to chemistry), quantum mechanics, nuclear and particle physics, plasma physics, remote sensing, biomolecular interactions, surface profiling, microfluidics, mechanical stress/strain measurement, velocimetry, and optometry.
Probe-based confocal laser endomicroscopy combines the slender camera-toting probe traditionally snaked down the throat to view the insides of organs (an endoscope) with a laser that lights up tissues, and sensors that analyze the reflected fluorescent patterns. It offers a microscopic view of living tissues instead of fixed ones. Confocal laser endomicroscopy (CLE) is a novel in vivo imaging technique that can provide real-time optical biopsies in the evaluation of pancreaticobiliary strictures and pancreatic cystic lesions (PCLs), both of which are plagued by low sensitivities of routine evaluation techniques. Compared to pathology alone, CLE is associated with a higher sensitivity and accuracy for the evaluation of indeterminate pancreaticobiliary strictures. CLE has the ability to determine the malignant potential of PCLs. As such, CLE can increase the diagnostic yield of endoscopic retrograde cholangiopancreatography and endoscopic ultrasound, reducing the need for repeat procedures. It has been shown to be safe, with an adverse event rate of ≤1%. Published literature regarding its cost-effectiveness is needed.
Contrast Agent is a substance used to increase the contrast of structures or fluids within the body in medical imaging. Contrast agents absorb or alter external electromagnetism or ultrasound, which is different from radiopharmaceuticals, which emit radiation themselves. In x-rays, contrast agents enhance the radiodensity in a target tissue or structure. In MRIs, contrast agents shorten (or in some instances increase) the relaxation times of nuclei within body tissues in order to alter the contrast in the image. Contrast agents are commonly used to improve the visibility of blood vessels and the gastrointestinal tract. Several types of contrast agent are in use in medical imaging and they can roughly be classified based on the imaging modalities where they are used. Most common contrast agents work based on X-ray attenuation and magnetic resonance signal enhancement.
Imaging Machines (EEG) - Microscopes (science tools)
Radiopharmaceutical are a group of pharmaceutical drugs which have radioactivity. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is different from contrast media which absorb or alter external electromagnetism or ultrasound. Radiopharmacology is the branch of pharmacology that specializes in these agents. The main group of these compounds are the radiotracers used to diagnose dysfunction in body tissues. While not all medical isotopes are radioactive, radiopharmaceuticals are the oldest and still most common such drugs.
Radioactive Tracer is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. Radioisotopes of hydrogen, carbon, phosphorus, sulfur, and iodine have been used extensively to trace the path of biochemical reactions. A radioactive tracer can also be used to track the distribution of a substance within a natural system such as a cell or tissue, or as a flow tracer to track fluid flow. Radioactive tracers are also used to determine the location of fractures created by hydraulic fracturing in natural gas production. Radioactive tracers form the basis of a variety of imaging systems, such as, PET scans, SPECT scans and technetium scans. Radiocarbon dating uses the naturally occurring carbon-14 isotope as an isotopic label.
Radionuclide is an atom that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are powerful enough to liberate an electron from another atom.
Scientists invent a new type of microscope that can see through an intact skull. The microscope uses a combination of hardware and software-based adaptive optics to reconstruct object image. Non-invasive microscopic techniques such as optical coherence microscopy and two-photon microscopy are commonly used for in vivo imaging of living tissues. When light passes through turbid materials such as biological tissues, two types of light are generated: ballistic photons and multiply scattered photons. The ballistic photons travel straight through the object without experiencing any deflection and hence is used to reconstruct the object image. On the other hand, the multiply scattered photons are generated via random deflections as the light passes through the material and show up as speckle noise in the reconstructed image. As the light propagates through increasing distances, the ratio between multiply scattered and ballistic photons increases drastically, thereby obscuring the image information. In addition to the noise generated by the multiply scattered light, optical aberration of ballistic light also causes contrast reduction and image blur during the image reconstruction process.
Everywhere and Nowhere at Once
 Observer Effect refers to changes that the act of
observation will make on a phenomenon
being observed. The act of
observing something could have an effect on what you are observing,
and trying to measure something can have an effect on what you are trying
to measure because the instruments can have an effect. This is often the
result of instruments that, by necessity, alter the state of what they
measure in some manner. A commonplace example is checking the pressure in
an automobile tire; this is difficult to do without letting out some of
the air, thus changing the pressure. Furthermore, it is not possible to
see any object without light hitting the object, and causing it to emit
light; while this may seem negligible, the object still experiences a
change. This effect can be observed in many domains of physics and can
often be reduced to insignificance by using better instruments or
observation techniques. In quantum mechanics, there is a common
misconception that it is the mind of a conscious observer
that causes the observer effect in quantum processes. It is rooted in a
misunderstanding of the quantum wave function ψ and the quantum
measurement process.
Observer Effect refers to changes that the act of
observation will make on a phenomenon
being observed. The act of
observing something could have an effect on what you are observing,
and trying to measure something can have an effect on what you are trying
to measure because the instruments can have an effect. This is often the
result of instruments that, by necessity, alter the state of what they
measure in some manner. A commonplace example is checking the pressure in
an automobile tire; this is difficult to do without letting out some of
the air, thus changing the pressure. Furthermore, it is not possible to
see any object without light hitting the object, and causing it to emit
light; while this may seem negligible, the object still experiences a
change. This effect can be observed in many domains of physics and can
often be reduced to insignificance by using better instruments or
observation techniques. In quantum mechanics, there is a common
misconception that it is the mind of a conscious observer
that causes the observer effect in quantum processes. It is rooted in a
misunderstanding of the quantum wave function ψ and the quantum
measurement process. Double Slit Experiment - Correlation does not Imply Causation.
Observational Error is the difference between a measured value of a quantity and its true value. In statistics, an error is not a "mistake". Variability is an inherent part of the results of measurements and of the measurement process. Measurement errors can be divided into two components: Random error and systematic error. Random errors are errors in measurement that lead to measurable values being inconsistent when repeated measurements of a constant attribute or quantity are taken. Systematic errors are errors that are not determined by chance but are introduced by an inaccuracy (involving either the observation or measurement process) inherent to the system. Systematic error may also refer to an error with a non-zero mean, the effect of which is not reduced when observations are averaged. Observer Errors can happen when people know they're being watched or videotaped, they will sometimes change their behavior.
Probe Effect is unintended alteration in system behavior caused by measuring that system. In code profiling and performance measurements, the delays introduced by insertion/removal of code instrumentation may result in a non-functioning application, or unpredictable behavior.
Born Rule is a key postulate of quantum mechanics which gives the probability that a measurement of a quantum system will yield a given result. In its simplest form, it states that the probability density of finding a particle at a given point is proportional to the square of the magnitude of the particle's wavefunction at that point.
Observer Effect in information technology is the impact on the behavior of a computer process caused by the act of observing the process while it is running. This effect is a manifestation of the uncertainty principle in information technology. The uncertainty principle is attributed to Werner Heisenberg and was originally referring to quantum mechanics.
Heisenbug is a software bug that seems to disappear or alter its behavior when one attempts to study it.
Uncertainty Principle also known as Heisenberg's uncertainty principle, is any of a variety of mathematical inequalities asserting a fundamental limit to the precision with which certain pairs of physical properties of a particle, known as complementary variables, such as position x and momentum p, can be known. Chaos Theory.
Heisenberg Uncertainty Principle states that there is an absolute limit on the combined accuracy of certain pairs of simultaneous, related measurements, especially that of the position and momentum of a particle. Originally posited as a problem of measurement, it was soon refined as an inherent property of the universe. Refraction and Diffraction of Light - Wave or Partial?
Electrons are waves and only look like particles when we look at them or try to measure them. Schrödinger Equation Superposition.
Measurement Problem in quantum mechanics is the problem of how (or whether) wave function collapse occurs. The inability to observe such a collapse directly has given rise to different interpretations of quantum mechanics and poses a key set of questions that each interpretation must answer. The wave function in quantum mechanics evolves deterministically according to the Schrödinger equation as a linear superposition of different states. However, actual measurements always find the physical system in a definite state. Any future evolution of the wave function is based on the state the system was discovered to be in when the measurement was made, meaning that the measurement "did something" to the system that is not obviously a consequence of Schrödinger evolution. The measurement problem is describing what that "something" is, how a superposition of many possible values becomes a single measured value. To express matters differently (paraphrasing Steven Weinberg), the Schrödinger wave equation determines the wave function at any later time. If observers and their measuring apparatus are themselves described by a deterministic wave function, why can we not predict precise results for measurements, but only probabilities? As a general question: How can one establish a correspondence between quantum and classical reality?
Spooky Action at a Distance is the concept that an object can be moved, changed, or otherwise affected without being physically touched (as in mechanical contact) by another object. That is, it is the nonlocal interaction of objects that are separated in space. Quantum Entanglement.
Complementarity in physics is both a theoretical and an experimental result of quantum mechanics, also referred to as principle of complementarity. It holds that objects have complementary properties which cannot all be observed or measured simultaneously. The complementarity principle was formulated by Niels Bohr, a leading founder of quantum mechanics. Examples of complementary properties that Bohr considered: Position and momentum. Energy and duration. Spin on different axes. Wave and particle. Value of a field and its change (at a certain position). Entanglement and coherence.
Relativity & The Equivalence of Reference Frames - Breakthrough Junior Challenge 2017 (youtube)
Smart atomic cloud solves Heisenberg's observation problem
Wheeler's Delayed Choice Experiment attempts to decide whether light somehow "senses" the experimental apparatus in the double-slit experiment, it will travel through and adjusts its behavior to fit by assuming the appropriate determinate state for it, or whether light remains in an indeterminate state, neither wave nor particle until measured. Wheeler's delayed-choice experiment is actually several thought experiments in quantum physics.
Counterfactual Quantum Computation is a method of inferring the result of a computation without actually running a quantum computer otherwise capable of actively performing that computation.
Electro-Optic Modulator is an optical device in which a signal-controlled element exhibiting the electro-optic effect is used to modulate a beam of light. The modulation may be imposed on the phase, frequency, amplitude, or polarization of the beam. Modulation bandwidths extending into the gigahertz range are possible with the use of laser-controlled modulators.
Mach–Zehnder Interferometer is a device used to determine the relative phase shift variations between two collimated beams derived by splitting light from a single source. The interferometer has been used, among other things, to measure phase shifts between the two beams caused by a sample or a change in length of one of the paths. Ludwig Zehnder (wiki).
Sagnac Effect is a phenomenon encountered in interferometry that is elicited by rotation. The Sagnac effect manifests itself in a setup called a ring interferometer. A beam of light is split and the two beams are made to follow the same path but in opposite directions. On return to the point of entry the two light beams are allowed to exit the ring and undergo interference. The relative phases of the two exiting beams, and thus the position of the interference fringes, are shifted according to the angular velocity of the apparatus. In other words, when the interferometer is at rest with respect to the earth, the light travels at a constant speed. However, when the interferometer system is spun, one beam of light will slow with respect to the other beam of light. A gimbal mounted mechanical gyroscope remains pointing in the same direction after spinning up, and thus can be used as a rotational reference for an inertial navigation system. With the development of so-called laser gyroscopes and fiber optic gyroscopes based on the Sagnac effect, the bulky mechanical gyroscope is replaced by one having no moving parts in many modern inertial navigation systems. The principles behind the two devices are different, however. A conventional gyroscope relies on the principle of conservation of angular momentum whereas the sensitivity of the ring interferometer to rotation arises from the invariance of the speed of light for all inertial frames of reference.
Transactional interpretation takes the psi and psi* wave functions of the standard quantum formalism to be retarded (forward in time) and advanced (backward in time) waves that form a quantum interaction as a Wheeler–Feynman handshake or transaction.
Neutrinos' Metamorphosis
De Broglie-Bohm Theory is an interpretation of quantum theory. In addition to a wave function on the space of all possible configurations, it also postulates an actual configuration that exists even when unobserved.
Doppler Effect is the change in frequency or wavelength of a wave (or other periodic event) for an observer moving relative to its source. It is named after the Austrian physicist Christian Doppler, who proposed it in 1842 in Prague. It is commonly heard when a vehicle sounding a siren or horn approaches, passes, and recedes from an observer. Compared to the emitted frequency, the received frequency is higher during the approach, identical at the instant of passing by, and lower during the recession.
Bouncing-Droplet Experiments
The Pilot-Wave Dynamics of Walking Droplets (youtube)
Schrodinger's Cat is a thought experiment, sometimes described as a paradox, devised by Austrian physicist Erwin Schrödinger in 1935. It illustrates what he saw as the problem of the Copenhagen interpretation of quantum mechanics applied to everyday objects. The scenario presents a cat that may be simultaneously both alive and dead, a state known as a quantum superposition, as a result of being linked to a random subatomic event that may or may not occur. The thought experiment is also often featured in theoretical discussions of the interpretations of quantum mechanics. Schrödinger coined the term Verschränkung (entanglement) in the course of developing the thought experiment. Wave Function.
Observation Flaws (Psychology) - Paranormal - Free Will - A persons thoughts can effect a water drop.
Implicate and Explicate Order is used to describe two different frameworks for understanding the same phenomenon or aspect of reality. In particular, the concepts were developed in order to explain the bizarre behavior of subatomic particles which quantum physics struggles to explain. In Bohm's Wholeness and the Implicate Order, he used these notions to describe how the appearance of such phenomena might appear differently, or might be characterized by, varying principal factors, depending on contexts such as scales. The implicate (also referred to as the "enfolded") order is seen as a deeper and more fundamental order of reality. In contrast, the explicate or "unfolded" order include the abstractions that humans normally perceive. As he wrote: In the enfolded [or implicate] order, space and time are no longer the dominant factors determining the relationships of dependence or independence of different elements. Rather, an entirely different sort of basic connection of elements is possible, from which our ordinary notions of space and time, along with those of separately existent material particles, are abstracted as forms derived from the deeper order. These ordinary notions in fact appear in what is called the "explicate" or "unfolded" order, which is a special and distinguished form contained within the general totality of all the implicate orders (Bohm 1980, p. xv).
Hidden-Variable Theory are proposals to provide deterministic explanations of quantum mechanical phenomena, through the introduction of unobservable hypothetical entities. The existence of indeterminacy for some measurements is assumed as part of the mathematical formulation of quantum mechanics; moreover, bounds for indeterminacy can be expressed in a quantitative form by the Heisenberg uncertainty principle. As per its mathematical formulation, quantum mechanics is non-deterministic, meaning that it generally does not predict the outcome of any measurement with certainty. Instead, it indicates what the probabilities of the outcomes are, with the indeterminism of observable quantities constrained by the uncertainty principle. The question arises whether there might be some deeper reality hidden beneath quantum mechanics, to be described by a more fundamental theory that can always predict the outcome of each measurement with certainty: if the exact properties of every subatomic particle were known the entire system could be modeled exactly using deterministic physics similar to classical physics. In other words, it is conceivable that quantum mechanics is an incomplete description of nature. The designation of variables as underlying "hidden" variables depends on the level of physical description (so, for example, "if a gas is described in terms of temperature, pressure, and volume, then the velocities of the individual atoms in the gas would be hidden variables"). Physicists supporting De Broglie–Bohm theory maintain that underlying the observed probabilistic nature of the universe is a deterministic objective foundation/property—the hidden variable. Others, however, believe that there is no deeper deterministic reality in quantum mechanics. A lack of a kind of realism (understood here as asserting independent existence and evolution of physical quantities, such as position or momentum, without the process of measurement) is crucial in the Copenhagen interpretation. Realistic interpretations (which were already incorporated, to an extent, into the physics of Feynman), on the other hand, assume that particles have certain trajectories. Under such view, these trajectories will almost always be continuous, which follows both from the finitude of the perceived speed of light ("leaps" should rather be precluded) and, more importantly, from the principle of least action, as deduced in quantum physics by Dirac. But continuous movement, in accordance with the mathematical definition, implies deterministic movement for a range of time arguments; and thus realism is, under modern physics, one more reason for seeking (at least certain limited) determinism and thus a hidden-variable theory (especially that such theory exists: see De Broglie–Bohm interpretation). Although determinism was initially a major motivation for physicists looking for hidden-variable theories, non-deterministic theories trying to explain what the supposed reality underlying the quantum mechanics formalism looks like are also considered hidden-variable theories; for example Edward Nelson's stochastic mechanics. "God does not play dice" In June 1926, Max Born published a paper, "Zur Quantenmechanik der Stoßvorgänge" ("Quantum Mechanics of Collision Phenomena") in the scientific journal Zeitschrift für Physik, in which he was the first to clearly enunciate the probabilistic interpretation of the quantum wave function, which had been introduced by Erwin Schrödinger earlier in the year. Born concluded the paper as follows: Here the whole problem of determinism comes up. From the standpoint of our quantum mechanics there is no quantity which in any individual case causally fixes the consequence of the collision; but also experimentally we have so far no reason to believe that there are some inner properties of the atom which conditions a definite outcome for the collision. Ought we to hope later to discover such properties ... and determine them in individual cases? Or ought we to believe that the agreement of theory and experiment—as to the impossibility of prescribing conditions for a causal evolution—is a pre-established harmony founded on the nonexistence of such conditions? I myself am inclined to give up determinism in the world of atoms. But that is a philosophical question for which physical arguments alone are not decisive.
Length Contraction is the phenomenon of a decrease in length of an object as measured by an observer who is traveling at any non-zero velocity relative to the object. Space time.
David Bohm was an American scientist who has been described as one of the most significant theoretical physicists of the 20th century and who contributed unorthodox ideas to quantum theory, neuropsychology and the philosophy of mind.
Richard Feynman was an American theoretical physicist known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics, and the physics of the superfluidity of supercooled liquid helium, as well as in particle physics for which he proposed the parton model. For his contributions to the development of quantum electrodynamics, Feynman, jointly with Julian Schwinger and Sin'ichirō Tomonaga, received the Nobel Prize in Physics in 1965.
Correspondence Problem refers to the problem of ascertaining which parts of one image correspond to which parts of another image, where differences are due to movement of the camera, the elapse of time, and/or movement of objects in the photos.
Coherence in physics states that two wave sources are perfectly coherent if they have a constant phase difference and the same frequency. It is an ideal property of waves that enables stationary (i.e. temporally and spatially constant) interference. It contains several distinct concepts, which are limiting cases that never quite occur in reality but allow an understanding of the physics of waves, and has become a very important concept in quantum physics. More generally, coherence describes all properties of the correlation between physical quantities of a single wave, or between several waves or wave packets.
Electromagnetic Field - Light - Color - Hertz - Sound - Quantum - Reality - Spatial Intelligence.
Widely used engineering technique has unintended consequences. Focused Ion Beam can in fact dramatically alter the material’s structural identity. A FIB setup is a scientific instrument that resembles a scanning electron microscope (SEM). However, while the SEM uses a focused beam of electrons to image the sample in the chamber, a FIB setup uses a Focused beam of ions instead. FIB can also be incorporated in a system with both electron and ion beam columns, allowing the same feature to be investigated using either of the beams. FIB should not be confused with using a beam of focused ions for direct write lithography (such as in proton beam writing). These are generally quite different systems where the material is modified by other mechanisms. PhysClips Waves and Sound.
Perfect transmission through barrier using sound. A research team has for the first time experimentally proved a century old quantum theory that relativistic particles can pass through a barrier with 100% transmission. The perfect transmission of sound through a barrier is difficult to achieve, if not impossible based on our existing knowledge. This is also true with other energy forms such as light and heat.
Leggett-Garg Inequality is a mathematical inequality fulfilled by all macrorealistic physical theories. Here, macrorealism (macroscopic realism) is a classical worldview defined by the conjunction of two postulates: Macrorealism per se: "A macroscopic object, which has available to it two or more macroscopically distinct states, is at any given time in a definite one of those states." Noninvasive measurability: "It is possible in principle to determine which of these states the system is in without any effect on the state itself, or on the subsequent system dynamics."
Life is like a simulation because humans understand how simulations work and see the similarities in life. This does not mean that life is a simulation. But what it does mean is that we are getting closer to fully understanding how our universe works, and that we have more controls than we ever dreamed about having. When people ask the wrong questions, they can easily make inaccurate assumptions. God Science: Episode One - The Simulation Hypothesis (youtube)
Does matter create mind or does mind create matter? Both. Matter creates the mind and the mind creates matter.
Mind & Matter - Mind over Matter...as a Matter of Fact...the Mind drives the Mass.
Corporeal is having material or physical form or substance. Affecting or characteristic of the body as opposed to the mind or spirit.
Dualism is the position that mental phenomena are, in some respects, non-physical, or that the mind and body are not identical. Thus, it encompasses a set of views about the relationship between mind and matter, and between subject and object, and is contrasted with other positions, such as physicalism and enactivism, in the mind–body problem.
Waves
 Wave is an
oscillation accompanied by a transfer of energy
that travels through a medium such
as space or
mass. Wave is a
pattern
of movement that
goes up and down or back and forth. Frequency refers to the addition of
time. Wave
motion transfers energy from one point to another, which displace
particles of the transmission medium–that is, with little or no associated
mass transport. Waves consist, instead, of
oscillations or
vibrations (of
a physical quantity), around almost fixed locations. A wave is a
disturbance that transfers energy through matter or space. There are two
main types of waves.
Mechanical waves propagate through a medium, and the substance of this
medium is deformed. Restoring forces then reverse the deformation. For
example, sound waves propagate via air molecules colliding with their
neighbors. When the molecules collide, they also bounce away from each
other (a restoring force). This keeps the molecules from continuing to
travel in the direction of the wave. The second main type, electromagnetic
waves, do not require a medium. Instead, they consist of periodic
oscillations of electrical and magnetic fields originally generated by
charged particles, and can therefore travel through a
vacuum. These types
vary in wavelength, and include radio waves, microwaves, infrared
radiation, visible light, ultraviolet radiation, X-rays and gamma rays.
Waves are described by a wave equation which sets out how the disturbance
proceeds over time. The mathematical form of this equation varies
depending on the type of wave. Further, the behavior of particles in
quantum mechanics are described by waves. In addition, gravitational waves
also travel through space, which are a result of a vibration or movement
in gravitational fields. A wave can be transverse, where a disturbance
creates oscillations that are perpendicular to the propagation of energy
transfer, or longitudinal: the oscillations are parallel to the direction
of energy propagation. While mechanical waves can be both transverse and
longitudinal, all electromagnetic waves are transverse in free space.
Noise.
Wave is an
oscillation accompanied by a transfer of energy
that travels through a medium such
as space or
mass. Wave is a
pattern
of movement that
goes up and down or back and forth. Frequency refers to the addition of
time. Wave
motion transfers energy from one point to another, which displace
particles of the transmission medium–that is, with little or no associated
mass transport. Waves consist, instead, of
oscillations or
vibrations (of
a physical quantity), around almost fixed locations. A wave is a
disturbance that transfers energy through matter or space. There are two
main types of waves.
Mechanical waves propagate through a medium, and the substance of this
medium is deformed. Restoring forces then reverse the deformation. For
example, sound waves propagate via air molecules colliding with their
neighbors. When the molecules collide, they also bounce away from each
other (a restoring force). This keeps the molecules from continuing to
travel in the direction of the wave. The second main type, electromagnetic
waves, do not require a medium. Instead, they consist of periodic
oscillations of electrical and magnetic fields originally generated by
charged particles, and can therefore travel through a
vacuum. These types
vary in wavelength, and include radio waves, microwaves, infrared
radiation, visible light, ultraviolet radiation, X-rays and gamma rays.
Waves are described by a wave equation which sets out how the disturbance
proceeds over time. The mathematical form of this equation varies
depending on the type of wave. Further, the behavior of particles in
quantum mechanics are described by waves. In addition, gravitational waves
also travel through space, which are a result of a vibration or movement
in gravitational fields. A wave can be transverse, where a disturbance
creates oscillations that are perpendicular to the propagation of energy
transfer, or longitudinal: the oscillations are parallel to the direction
of energy propagation. While mechanical waves can be both transverse and
longitudinal, all electromagnetic waves are transverse in free space.
Noise. Electricity - Wireless Energy - Tone Generator
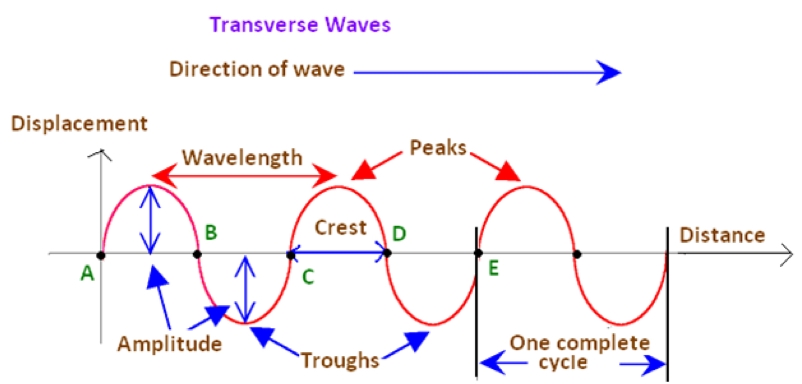 Waveform is the
shape and form of a signal such as a wave moving in a physical medium or
an abstract representation. In many cases the medium in which the wave
propagates does not permit a direct observation of the true form. In these
cases, the term "waveform" refers to the shape of a graph of the varying
quantity against time. An instrument called an oscilloscope can be used to
pictorially represent a wave as a repeating image on a screen. To be more
specific, a waveform is depicted by a graph that shows the changes in a
recorded signal's amplitude over the duration of recording. The amplitude
of the signal is measured on the y {\displaystyle y} y-axis (vertical),
and time on the x {\displaystyle x} x-axis (horizontal).
Fields.
Waveform is the
shape and form of a signal such as a wave moving in a physical medium or
an abstract representation. In many cases the medium in which the wave
propagates does not permit a direct observation of the true form. In these
cases, the term "waveform" refers to the shape of a graph of the varying
quantity against time. An instrument called an oscilloscope can be used to
pictorially represent a wave as a repeating image on a screen. To be more
specific, a waveform is depicted by a graph that shows the changes in a
recorded signal's amplitude over the duration of recording. The amplitude
of the signal is measured on the y {\displaystyle y} y-axis (vertical),
and time on the x {\displaystyle x} x-axis (horizontal).
Fields.Pulse in electronics is a sharp transient wave in the normal electrical state, or a series of such transients. The rhythmic contraction and expansion of the arteries with each beat of the heart. The rate at which the heart beats; usually measured to obtain a quick evaluation of a person's health. Pulse Rate.
Standing Wave is a wave in which its peaks (or any other point on the wave) do not move spatially. The amplitude of the wave at a point in space may vary with time, but its phase remains constant. The locations at which the amplitude is minimum are called nodes, and the locations where the amplitude is maximum are called antinodes.
 Node in physics is a point along a standing wave where the wave has
minimum amplitude. For instance, in a vibrating guitar string, the ends of
the string are nodes. By changing the position of the end node through
frets, the guitarist changes the effective length of the vibrating string
and thereby the note played. The opposite of a node is an anti-node, a
point where the amplitude of the standing wave is a maximum. These occur
midway between the nodes.
Node in physics is a point along a standing wave where the wave has
minimum amplitude. For instance, in a vibrating guitar string, the ends of
the string are nodes. By changing the position of the end node through
frets, the guitarist changes the effective length of the vibrating string
and thereby the note played. The opposite of a node is an anti-node, a
point where the amplitude of the standing wave is a maximum. These occur
midway between the nodes.
Sound Waves Makes Shapes - Slow Moving Waves in Giant Hanging Loops - Cool Science Experiment (youtube)
Arbitrary Waveform Generator is a piece of electronic test equipment used to generate electrical waveforms. These waveforms can be either repetitive or single-shot (once only) in which case some kind of triggering source is required (internal or external). The resulting waveforms can be injected into a device under test and analyzed as they progress through it, confirming the proper operation of the device or pinpointing a fault in it. Digital Pattern Generator.
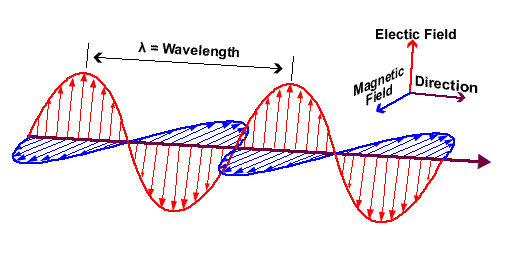 Function Generator is usually a piece of
electronic test equipment or
software used to generate different types of electrical waveforms over a
wide range of frequencies. Some of the most common waveforms produced by
the function generator are the sine, square, triangular and sawtooth
shapes. These waveforms can be either repetitive or single-shot (which
requires an internal or external trigger source). Integrated circuits used
to generate waveforms may also be described as function generator ICs.
Function Generator is usually a piece of
electronic test equipment or
software used to generate different types of electrical waveforms over a
wide range of frequencies. Some of the most common waveforms produced by
the function generator are the sine, square, triangular and sawtooth
shapes. These waveforms can be either repetitive or single-shot (which
requires an internal or external trigger source). Integrated circuits used
to generate waveforms may also be described as function generator ICs.
Electromagnetic waves have both electric and magnetic field components, which oscillate in phase perpendicular to each other and perpendicular to the direction of energy propagation, but they carry no electric charge themselves. The creation of all electromagnetic waves begins with an oscillating charged particle, which creates oscillating electric and magnetic fields. Once in motion, the electric and magnetic fields that a charged particle creates are self-perpetuating: time-dependent changes in one field (electric or magnetic) produce the other. Massless.
 Wave
Function in quantum physics is a mathematical
description of the quantum state of a system. The wave function is a
complex-valued probability amplitude, and the probabilities for the
possible results of measurements made on the system can be derived from
it. The wave function is a function of the degrees of freedom
corresponding to some maximal set of commuting observables. Once such
a representation is chosen, the wave function can be derived from the
quantum state.
The most common symbols for a wave function are the Greek letters ψ or
Ψ (lower-case and capital psi, respectively).
Wave
Function in quantum physics is a mathematical
description of the quantum state of a system. The wave function is a
complex-valued probability amplitude, and the probabilities for the
possible results of measurements made on the system can be derived from
it. The wave function is a function of the degrees of freedom
corresponding to some maximal set of commuting observables. Once such
a representation is chosen, the wave function can be derived from the
quantum state.
The most common symbols for a wave function are the Greek letters ψ or
Ψ (lower-case and capital psi, respectively).
Coherence is when two wave sources are perfectly coherent if they have a constant phase difference and the same frequency, and the same waveform. Coherence is an ideal property of waves that enables stationary (i.e. temporally and spatially constant) interference. It contains several distinct concepts, which are limiting cases that never quite occur in reality but allow an understanding of the physics of waves, and has become a very important concept in quantum physics. More generally, coherence describes all properties of the correlation between physical quantities of a single wave, or between several waves or wave packets.
Wave or Particle or Both
Wave Particle Duality is the concept that every elementary particle or quantic entity may be partly described in terms not only of particles, but also of waves. It expresses the inability of the classical concepts "particle" or "wave" to fully describe the behavior of quantum-scale objects. Observation Errors.
Double-Slit Experiment is a demonstration that light and matter can display characteristics of both classically defined waves and particles; moreover, it displays the fundamentally probabilistic nature of quantum mechanical phenomena. Electrons behave differently when they're observed.
Copenhagen Interpretation is an expression of the meaning of quantum mechanics that states that material objects, on a microscopic level, generally do not have definite properties prior to being measured, and quantum mechanics can only predict the probability distribution of a given measurement's possible results. The act of measurement affects the system, causing the set of probabilities to reduce to only one of the possible values immediately after the measurement. This feature is known as wave function collapse.
Wave Interference is a phenomenon in which two waves superpose to form a resultant wave of greater, lower, or the same amplitude. Interference usually refers to the interaction of waves that are correlated or coherent with each other, either because they come from the same source or because they have the same or nearly the same frequency. Interference effects can be observed with all types of waves, for example, light, radio, acoustic, surface water waves or matter waves. Interference Pattern.
Quantum Decoherence is the loss of quantum coherence. In quantum mechanics, particles such as electrons behave like waves and are described by a wavefunction. These waves can interfere, leading to the peculiar behaviour of quantum particles. As long as there exists a definite phase relation between different states, the system is said to be coherent. This coherence is a fundamental property of quantum mechanics, and is necessary for the functioning of quantum computers. However, when a quantum system is not perfectly isolated, but in contact with its surroundings, the coherence decays with time, a process called quantum decoherence. As a result of this process, the quantum behaviour is lost. Quantum Superposition.
Schrödinger Equation is a mathematical equation that describes the changes over time of a physical system in which quantum effects, such as wave–particle duality, are significant. The equation is a mathematical formulation for studying quantum mechanical systems. It is considered a central result in the study of quantum systems and its derivation was a significant landmark in developing the theory of quantum mechanics. Schrodinger Equation is a linear partial differential equation that describes the wave function or state function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of the subject. Schrodinger's Cat - Zero Point.
Probability Amplitude is a complex number used in describing the behaviour of systems. The modulus squared of this quantity represents a probability or probability density. Probability amplitudes provide a relationship between the wave function (or, more generally, of a quantum state vector) of a system and the results of observations of that system. Probability Wave. A quantum state of a particle or system, as characterized by a wave propagating through space, in which the square of the magnitude of the wave at any given point corresponds to the probability of finding the particle at that point.
Wave Function Collapse is said to occur when a wave function—initially in a superposition of several eigenstates—appears to reduce to a single eigenstate (by "observation"). It is the essence of measurement in quantum mechanics and connects the wave function with classical observables like position and momentum.
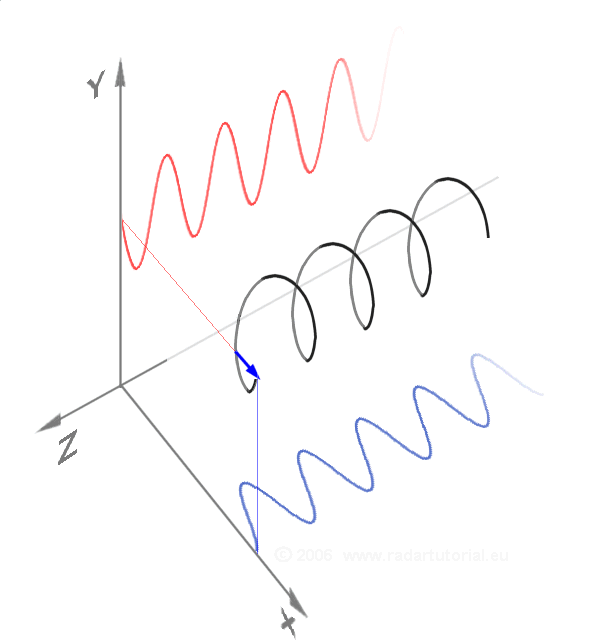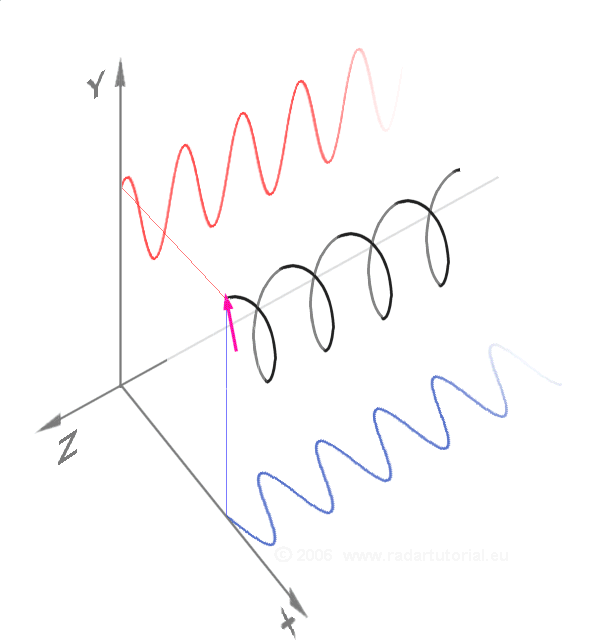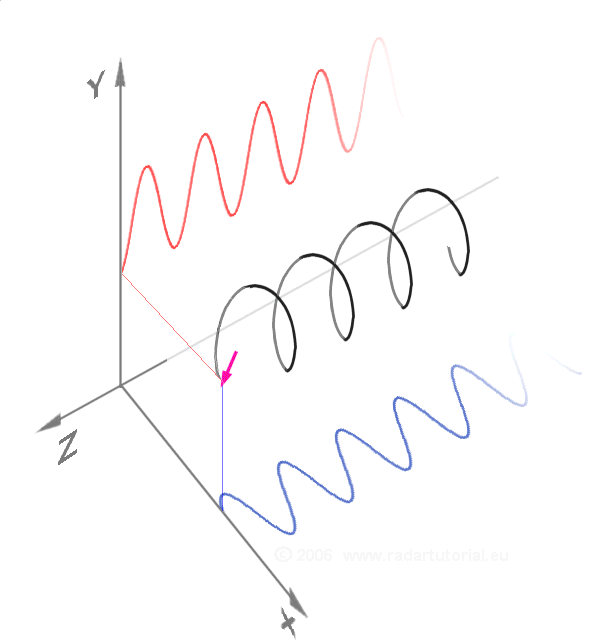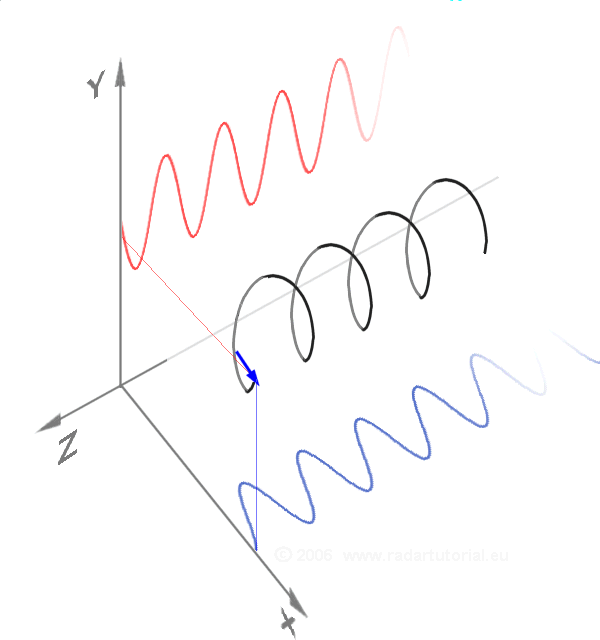 Pilot Wave was the first known example of a
hidden variable theory, where the state of a physical system, as
formulated by quantum mechanics, does not give a complete description for
the system; i.e., that quantum mechanics is ultimately incomplete, and
that a complete theory would provide descriptive categories to account for
all observable behavior and thus avoid any indeterminism.
Pilot Wave was the first known example of a
hidden variable theory, where the state of a physical system, as
formulated by quantum mechanics, does not give a complete description for
the system; i.e., that quantum mechanics is ultimately incomplete, and
that a complete theory would provide descriptive categories to account for
all observable behavior and thus avoid any indeterminism.De Broglie - Bohm theory is an interpretation of quantum mechanics. In addition to a wavefunction on the space of all possible configurations, it also postulates an actual configuration that exists even when unobserved. The evolution over time of the configuration (that is, the positions of all particles or the configuration of all fields) is defined by a guiding equation that is the nonlocal part of the wave function. The evolution of the wave function over time is given by the Schrödinger equation. (also known as the pilot wave theory, Bohmian mechanics, Bohm's interpretation, and the causal interpretation).
Faraday Wave are nonlinear standing waves that appear on liquids enclosed by a vibrating receptacle. When the vibration frequency exceeds a critical value, the flat hydrostatic surface becomes unstable. This is known as the Faraday instability.
Polarization Waves is a parameter applying to waves that specifies the geometrical orientation of the oscillation. Electromagnetic waves such as light exhibit multiple polarizations, as do gravitational waves and sound waves in solids. On the other hand, sound waves in a gas or liquid only oscillate in the wave's direction of propagation, and the oscillation of ocean waves is always in the vertical direction. In these cases one doesn't normally speak of "polarization" since the oscillation's direction is not in question.
Order and Disorder in physics designates the presence or absence of some symmetry or correlation in a many-particle system.
Quantum Superposition
Zero-Point Energy is the lowest possible energy that a quantum mechanical system may have, i.e. it is the energy of the system's ground state. Zero-point energy can have several different types of context, e.g. it may be the energy associated with the ground state of an atom, a subatomic particle or even the quantum vacuum itself. Perpetual.
Principle Vibration. Nothing rests; everything moves; everything vibrates. The Principle of Vibration states that nothing in the universe is at rest, everything vibrates, everything is in motion. Vibration is in everything, from the tiniest molecule to the biggest rock, in physical and biological systems, we find vibration in matter, energy, light and sound. In physics vibration is often called oscillation - either a movement back and fro as in the swing of a pendulum or random vibrations as are exhibited in the Brownian Movement. It can be described by three factors: the amplitude (size) , the frequency (rate) and the phase (timing). Occultists state that differences in rate and character of vibration determine the different planes of being, seeing the highest plane as that with the highest rate of vibration. Also, every mental and/or emotional state has its own rate of vibration the knowledge of which could enable a skilled person to influence at will. The Principle of Vibration is closely connected to the Principle of Polarity and the Principle of Rhythm.
Principle of Rhythm expresses the idea that in everything there is manifested a measured motion, a to and from, a flow and inflow, a swing backward and forward, a pendulum-like movement.
Principle of Polarity embodies the idea that everything is dual, everything has two poles, and everything has its opposite. All manifested things have two sides, two aspects, or two poles.
Law of Vibration states that everything is energy and the energy is vibrating at a certain frequency.
Motion is manifest in everything in the Universe, that nothing rests, and everything moves, vibrates and circles.
Things Come In Waves. You can ride the wave, you can let the wave pass through you, you can go against the wave, and you can even make waves yourself. Waves are patterns, but not all patterns are good. If bad things repeat themselves, then you have problems. If good things repeat themselves, then you have progress. If you make waves, make sure that they are good waves that send good vibrations.
"I like it when a surfer tells me that "Life is a Wave!" I like to reply and say that "Life is also a particle and not just a wave", so go for it dude, enjoy the ride."
Electromagnetic Spectrum
 Electromagnetic Spectrum is all
the known range of frequencies and their
linked wavelengths of
electromagnetic radiation and their respective wavelengths
and
photon energies. The
electromagnetic spectrum of an object has a different meaning, and is
instead the characteristic distribution of
electromagnetic radiation
emitted or absorbed by that particular object. The electromagnetic
spectrum extends from below the low frequencies used for modern
radio
communication to gamma radiation at the short-wavelength (high-frequency)
end, thereby covering wavelengths from thousands of kilometers down to a
fraction of the size of an atom. Visible light lies toward the shorter
end, with wavelengths from 400 to 700 nanometres. The limit for long
wavelengths is the size of the universe itself, while it is thought that
the short wavelength limit is in the vicinity of the Planck length. Until
the middle of the 20th century it was believed by most physicists that
this spectrum was infinite and continuous. Nearly all types of
electromagnetic radiation can be used for spectroscopy, to study and
characterize matter. Other technological uses are described under
electromagnetic radiation. (Maxwell's
equations predicted an infinite number of
frequencies of electromagnetic waves, all traveling at the speed of
light. The number of frequencies in the entire spectrum is the number 81
with 31 zeros after it).
Electromagnetic Spectrum is all
the known range of frequencies and their
linked wavelengths of
electromagnetic radiation and their respective wavelengths
and
photon energies. The
electromagnetic spectrum of an object has a different meaning, and is
instead the characteristic distribution of
electromagnetic radiation
emitted or absorbed by that particular object. The electromagnetic
spectrum extends from below the low frequencies used for modern
radio
communication to gamma radiation at the short-wavelength (high-frequency)
end, thereby covering wavelengths from thousands of kilometers down to a
fraction of the size of an atom. Visible light lies toward the shorter
end, with wavelengths from 400 to 700 nanometres. The limit for long
wavelengths is the size of the universe itself, while it is thought that
the short wavelength limit is in the vicinity of the Planck length. Until
the middle of the 20th century it was believed by most physicists that
this spectrum was infinite and continuous. Nearly all types of
electromagnetic radiation can be used for spectroscopy, to study and
characterize matter. Other technological uses are described under
electromagnetic radiation. (Maxwell's
equations predicted an infinite number of
frequencies of electromagnetic waves, all traveling at the speed of
light. The number of frequencies in the entire spectrum is the number 81
with 31 zeros after it). Humans can only see a small percentage of the entire electromagnetic spectrum with our eyes, but with technology we can see a lot of the wavelengths that are invisible to our eyes.
Visible Spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 700 nm. In terms of frequency, this corresponds to a band in the vicinity of 430–770 THz. The spectrum does not, however, contain all the colors that the human eyes and brain can distinguish. Unsaturated colors such as pink, or purple variations such as magenta, are absent, for example, because they can be made only by a mix of multiple wavelengths. Colors containing only one wavelength are also called pure colors or spectral colors. Visible wavelengths pass through the "optical window", the region of the electromagnetic spectrum that allows wavelengths to pass largely unattenuated through the Earth's atmosphere. An example of this phenomenon is that clean air scatters blue light more than red wavelengths, and so the midday sky appears blue. The optical window is also referred to as the "visible window" because it overlaps the human visible response spectrum. The near infrared or NIR window lies just out of the human vision, as well as the Medium Wavelength IR or MWIR window, and the Long Wavelength or Far Infrared or LWIR or FIR window, although other animals may experience them.
Optical Window is the optical portion of the electromagnetic spectrum that passes through the atmosphere all the way to the ground. Most EM wavelengths are blocked by the atmosphere, so this is like a window that lets through only a narrow selection of what is out there, though the sun is particularly active in the passed wavelengths. It is called "optical" because the wavelengths we can see are all in this range. The window runs from around 300 nanometers (ultraviolet-B) at the short end up into the range the eye can use, roughly 400–700 nm and continues up through the visual infrared to around 1100 nm, which is in the near-infrared range. There are also infrared and "radio windows" that transmit some infrared and radio waves. The radio window runs from about one centimeter to about eleven-meter waves. Optical window in medical physics, the optical window is the portion of the visible and infrared spectrum where living tissue absorbs relatively little light. This window runs approximately from 650 to 1200 nm. At shorter wavelengths, light is strongly absorbed by hemoglobin in blood, while at longer wavelengths water strongly absorbs infrared light. Optical window in optics, it means a (usually at least mechanically flat, sometimes optically flat, depending on resolution requirements) piece of transparent (for a wavelength range of interest, not necessarily for visible light) optical material that allows light into an optical instrument. A window is usually parallel and is likely to be anti-reflection coated, at least if it is designed for visible light. An optical window may be built into a piece of equipment (such as a vacuum chamber) to allow optical instruments to view inside that equipment.
Light - Colors - Eye Color - Electricity - Radio Waves
Full-Spectrum Light is light that covers the electromagnetic spectrum from infrared to near-ultraviolet, or all wavelengths that are useful to plant or animal life; in particular, sunlight is considered full spectrum, even though the solar spectral distribution reaching Earth changes with time of day, latitude, and atmospheric conditions. "Full-spectrum" is not a technical term when applied to an electrical light bulb. Rather, it implies that the product emulates some important quality of natural light. Products marketed as "full-spectrum" may produce light throughout the entire visible spectrum, but without producing an even spectral distribution. Some may not differ substantially from lights not marketed as full-spectrum.
Trichromacy is the condition of possessing three independent channels for conveying color information, derived from the three different cone types. Organisms with trichromacy are called trichromats.
Spectrum is a condition that is not limited to a specific set of values but can vary, without steps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors in visible light after passing through a prism. As scientific understanding of light advanced, it came to apply to the entire electromagnetic spectrum.
Emission Spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.
Atomic Spectrum is the spectrum of frequencies of electromagnetic radiation emitted or absorbed during transitions of electrons between energy levels within an atom. Each element has a characteristic spectrum by which it can be recognized.
Spectral Density describes the distribution of power into frequency components composing that signal. According to Fourier analysis any physical signal can be decomposed into a number of discrete frequencies, or a spectrum of frequencies over a continuous range. The statistical average of a certain signal or sort of signal (including noise) as analyzed in terms of its frequency content, is called its spectrum.
Spectrum Analyzer measures the magnitude of an input signal versus frequency within the full frequency range of the instrument. The primary use is to measure the power of the spectrum of known and unknown signals. The input signal that a spectrum analyzer measures is electrical; however, spectral compositions of other signals, such as acoustic pressure waves and optical light waves, can be considered through the use of an appropriate transducer. Optical spectrum analyzers also exist, which use direct optical techniques such as a monochromator to make measurements. Scopes.
Spectrometer is a scientific instrument originally used to split light into an array of separate colors, called a spectrum. Spectrometers were developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine chemical composition drove its advancement and continues to be one of their primary uses. Spectrometers are used in astronomy to analyze the chemical composition of stars and planets, and spectrometers gather data on the origin of the universe. The concept of a spectrometer now encompasses instruments that do not examine light. Spectrometers separate particles, atoms, and molecules by their mass, momentum, or energy. These types of spectrometers are used in chemical analysis and particle physics.
Spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. It is more specific than the general term electromagnetic spectroscopy in that spectrophotometry deals with visible light, near-ultraviolet, and near-infrared, but does not cover time-resolved spectroscopic techniques. Spectrophotometry uses photometers, known as spectrophotometers, that can measure a light beam's intensity as a function of its color (wavelength). Important features of spectrophotometers are spectral bandwidth (the range of colors it can transmit through the test sample), the percentage of sample-transmission, the logarithmic range of sample-absorption, and sometimes a percentage of reflectance measurement. A spectrophotometer is commonly used for the measurement of transmittance or reflectance of solutions, transparent or opaque solids, such as polished glass, or gases. Although many biochemicals are colored, as in, they absorb visible light and therefore can be measured by colorimetric procedures, even colorless biochemicals can often be converted to colored compounds suitable for chromogenic color-forming reactions to yield compounds suitable for colorimetric analysis. However they can also be designed to measure the diffusivity on any of the listed light ranges that usually cover around 200 nm - 2500 nm using different controls and calibrations. Within these ranges of light, calibrations are needed on the machine using standards that vary in type depending on the wavelength of the photometric determination.
Spectroscopy is the study of the interaction between matter and electromagnetic radiation or any interaction with radiative energy as a function of its wavelength or frequency. Spectroscopic data is often represented by an emission spectrum, a plot of the response of interest as a function of wavelength or frequency.
Ultraviolet is an electromagnetic radiation with a wavelength from 10 nm (30 PHz) to 400 nm (750 THz), shorter than that of visible light but longer than X-rays. UV radiation constitutes about 10% of the total light output of the Sun, and is thus present in sunlight. It is also produced by electric arcs and specialized lights such as mercury-vapor lamps, tanning lamps, and black lights. Although it is not considered an ionizing radiation because its photons lack the energy to ionize atoms, long-wavelength ultraviolet radiation can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. UV Index.
Spatial Intelligence
Magnetism
Acoustic Spectrum (sound)
A 100-year-old physics problem has been solved at EPFL. Researchers challenge a fundamental law and discover that more electromagnetic energy can be stored in wave-guiding systems than previously thought. Their trick was to create asymmetric resonant or wave-guiding systems using magnetic fields.
Can You See Me? FLIR T1K Thermal Imaging Camera (youtube)
Thermography are examples of infrared imaging science. Thermographic cameras usually detect radiation in the long-infrared range of the electromagnetic spectrum (roughly 9,000–14,000 nanometers or 9–14 µm) and produce images of that radiation, called thermograms. Since infrared radiation is emitted by all objects with a temperature above absolute zero according to the black body radiation law, thermography makes it possible to see one's environment with or without visible illumination. The amount of radiation emitted by an object increases with temperature; therefore, thermography allows one to see variations in temperature. When viewed through a thermal imaging camera, warm objects stand out well against cooler backgrounds; humans and other warm-blooded animals become easily visible against the environment, day or night. As a result, thermography is particularly useful to the military and other users of surveillance cameras.
Infrared is electromagnetic radiation with longer wavelengths than those of visible light, and is therefore invisible, although it is sometimes loosely called infrared light. It extends from the nominal red edge of the visible spectrum at 700 nanometers (frequency 430 THz), to 1000000 nm (300 GHz) (although people can see infrared up to at least 1050 nm in experiments. Most of the thermal radiation emitted by objects near room temperature is infrared. Like all EMR, IR carries radiant energy, and behaves both like a wave and like its quantum particle, the photon. Infrared Thermometer.
Infrared Spectroscopy involves the interaction of infrared radiation with matter. Infrared Microspectroscopy. IR spectroscopy is a widely used and versatile method for analysis at the molecular scale. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic techniques, it can be used to identify and study chemicals. Samples may be solid, liquid, or gas. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) to produce an infrared spectrum. An IR spectrum is essentially a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency or wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimeters (sometimes called wave numbers), with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to wave numbers in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. The infrared portion of the electromagnetic spectrum is usually divided into three regions; the near-, mid- and far- infrared, named for their relation to the visible spectrum. The higher-energy near-IR, approximately 14000–4000 cm−1 (0.8–2.5 μm wavelength) can excite overtone or harmonic vibrations. The mid-infrared, approximately 4000–400 cm−1 (2.5–25 μm) may be used to study the fundamental vibrations and associated rotational-vibrational structure. The far-infrared, approximately 400–10 cm−1 (25–1000 μm), lying adjacent to the microwave region, has low energy and may be used for rotational spectroscopy. The names and classifications of these subregions are conventions, and are only loosely based on the relative molecular or electromagnetic properties. New infrared imaging technique reveals molecular orientation of proteins in silk fibres.
Near-Infrared Spectroscopy is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum (from 780 nm to 2500 nm). Typical applications include medical and physiological diagnostics and research including blood sugar, pulse oximetry, functional neuroimaging, sports medicine, elite sports training, ergonomics, rehabilitation, neonatal research, brain computer interface, urology (bladder contraction), and neurology (neurovascular coupling). There are also applications in other areas as well such as pharmaceutical, food and agrochemical quality control, atmospheric chemistry, combustion research and astronomy.
Infrared Astronomical Satellite (IRAS)
Infrared Window is the overall dynamic property of the earth's atmosphere, taken as a whole at each place and occasion of interest, that lets some infrared radiation from the cloud tops and land-sea surface pass directly to space without intermediate absorption and re-emission, and thus without heating the atmosphere. It cannot be defined simply as a part or set of parts of the electromagnetic spectrum, because the spectral composition of window radiation varies greatly with varying local environmental conditions, such as water vapour content and land-sea surface temperature, and because few or no parts of the spectrum are simply not absorbed at all, and because some of the diffuse radiation is passing nearly vertically upwards and some is passing nearly horizontally. A large gap in the absorption spectrum of water vapor, the main greenhouse gas, is most important in the dynamics of the window. Other gases, especially carbon dioxide and ozone, partly block transmission. An atmospheric window is a dynamic property of the atmosphere, while the spectral window is a static characteristic of the electromagnetic radiative absorption spectra of many greenhouse gases, including water vapour. The atmospheric window tells what actually happens in the atmosphere, while the spectral window tells of one of the several abstract factors that potentially contribute to the actual concrete happenings in the atmosphere. Window radiation is radiation that passes through the atmospheric window, whereas non-window radiation is radiation that does not. Window wavelength radiation is radiation that, judging only from its wavelength, is likely to pass through the atmospheric window. The difference between window radiation and window wavelength radiation is that window radiation is an actual component of the radiation, determined by the full dynamics of the atmosphere, taking in all determining factors, while window wavelength radiation is merely theoretically potential, defined only by one factor, the wavelength.
Near-Infrared Window in Biological Tissue defines the range of wavelengths from 650 to 1350 nanometre (nm) where light has its maximum depth of penetration in tissue. Within the NIR window, scattering is the most dominant light-tissue interaction, and therefore the propagating light becomes diffused rapidly. Since scattering increases the distance travelled by photons within tissue, the probability of photon absorption also increases. Because scattering has weak dependence on wavelength, the NIR window is primarily limited by the light absorption of blood at short wavelengths and water at long wavelengths. The technique using this window is called NIRS. Medical imaging techniques such as fluorescence image-guided surgery often make use of the NIR window to detect deep structures.
GLEAM Data Sphere Animation Red indicates the lowest frequencies, green the middle frequencies and blue the highest frequencies. (video)
Chromoscope lets you explore our Galaxy the Milky Way and the distant Universe in a range of wavelengths from gamma-rays to the longest radio waves.
Electromagnetic Radiation in this range of wavelengths called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 700 nm. In terms of frequency, this corresponds to a band in the vicinity of 430–770 THz.
Terahertz - Wave of the future: Terahertz chips a new way of seeing through matter - 1 E-7 m (wiki)
Cellphone Radiation.
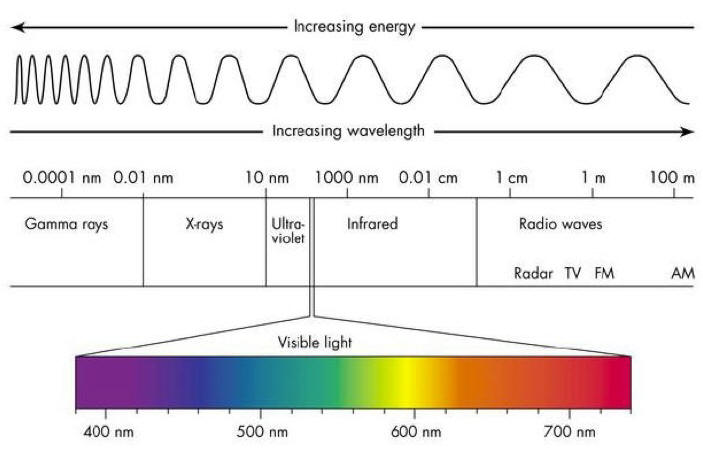
There are an uncountable infinity of possible wavelengths. In general the frequency spectrum for Electromagnetic (e.g light, radio, etc.) is continuous and thus between any two frequencies there are an uncountable infinity of possible frequencies (just as there are an uncountable number of numbers between 1 and 2). Brain Waves.
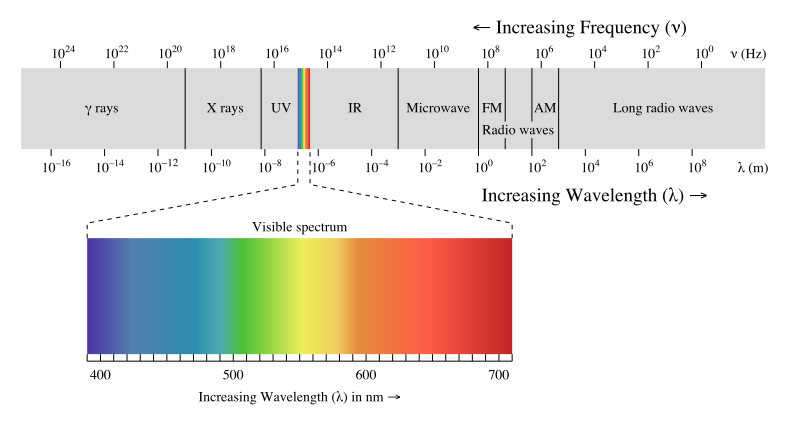
Higher Frequency as things get Smaller.
Graph Below is Reversed
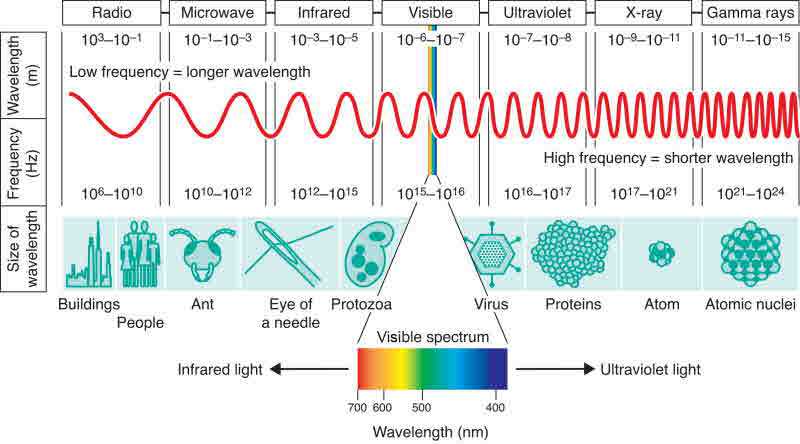
Radio Waves have frequencies as high as 300 gigahertz (GHz) to as low as 30 hertz (Hz). At 300 GHz, the corresponding wavelength is 1 mm, and at 30 Hz is 10,000 km. Like all other electromagnetic waves, radio waves travel at the speed of light in vacuum.
Ultrathin, flat lens resolves chirality and color Light can also be chiral. In chiral light, the direction of oscillation of the electromagnetic wave. Multispectral chiral lens.
Computers act like human brains because human brains made computers. This does not mean that machines can be humans?
"No one created math, math was discovered because math already existed in nature. And just because math exists in nature does not mean that all life is a calculation."
Videos about Physics
Minute Physics (youtube)Khan Physics (videos)
Physics Fun (youtube channel)
Open Letter to the President: Physics Education (youtube)
Nassim Haramein (youtube)
Tom Campbell (youtube)
Through the Wormhole (youtube)
6.2 Introduction to Atomic Structure (youtube)
khan Academy Electron Configurations (video)
Veritasium (youtube channel of science and engineering)
Science Videos and Films
Physics Teaching Resources
Physics ClassroomPhysics World
Physics
Institute of Physics
Physics 4 Kids
Tutor 4 Physics
Nordic Institute for Theoretical Physics
Physics Illinois.edu
Physics Stackexchange
Publications in Physics (wiki)
Adventure in Physics and Math by Edward Witten (pdf)