BK101
Knowledge Base
Chemistry
 Chemistry is the science
of matter; The branch of the natural sciences dealing with the
composition of substances and their
properties and reactions.
Chemistry is the science
of matter; The branch of the natural sciences dealing with the
composition of substances and their
properties and reactions. Biology - Elements - Carbon - Thermodynamics
Glossary of Chemistry Terms (wiki) - Types of Chemistry
Chemistry Tools - Science Equipment - Scopes - Microscopes
Khan Chemistry (videos) - ACS Reactions (youtube)
Chemistry Stack Exchange is a question and answer site for scientists.
Middle School Chemistry - Do it Yourself Chemistry (DIY)
 Chemist is a scientist trained in the study of chemistry.
Chemists study the composition of matter and its properties. Chemists
carefully describe the properties they study in terms of quantities,
with detail on the level of molecules and their component
atoms.
Chemists carefully measure substance proportions, reaction rates, and
other chemical properties. Drug
Research.
Chemist is a scientist trained in the study of chemistry.
Chemists study the composition of matter and its properties. Chemists
carefully describe the properties they study in terms of quantities,
with detail on the level of molecules and their component
atoms.
Chemists carefully measure substance proportions, reaction rates, and
other chemical properties. Drug
Research.Chemical is a substance produced by reactions or used in reactions involving atomic or molecular changes. A compound or a substance that has been purified or prepared, especially artificially. Material produced by or used in a reaction involving changes in atoms or molecules. Relating to or used in chemistry.
Chemical Substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be chemical elements, chemical compounds, ions or alloys. Carbon.
Substrate is the substance that is acted upon by an enzyme or ferment. A surface on which an organism grows or is attached. Any stratum or layer lying underneath another.
Antigen in immunology is any substance as a toxin or enzyme that stimulates an immune response in the body, especially the production of antibodies.
Mole is the unit of measurement for amount of substance in the International System of Units (SI). The unit is defined as the amount or sample of a chemical substance that contains as many constitutive particles, e.g., atoms, molecules, ions, electrons, or photons, as there are atoms in 12 grams of carbon-12 (12C), the isotope of carbon with standard atomic weight 12 by definition. This number is expressed by the Avogadro constant, which has a value of approximately 6.022140857×1023 mol−1. The mole is an SI base unit, with the unit symbol mol.
Chemical Property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity. Simply speaking, chemical properties cannot be determined just by viewing or touching the substance; the substance's internal structure must be affected greatly for its chemical properties to be investigated. When a substance goes under a chemical reaction, the properties will change drastically, resulting in chemical change. However, a catalytic property would also be a chemical property. Temperature.
Chemical Composition is the identity, and relative number, of the elements that make up any particular compound. It refers to the arrangement, type, and ratio of atoms in molecules of chemical substances. Chemical composition varies when chemicals are added or subtracted from a substance, when the ratio of substances changes, or when other chemical changes occur in chemicals. The chemical composition of a pure substance corresponds to the relative amounts of the elements that constitute the substance itself. It can be expressed by the empirical formula. For example the formula for water is H2O: this means that each molecule is constituted by 2 atoms of hydrogen (H) and 1 atom of oxygen (O). The chemical composition of a mixture can be defined as the distribution of the single substances that constitute the mixture, called "components". In other words, it is defined giving the concentration of each component. Because there are different ways to define the concentration of a component, as a consequence there are also different ways to define the composition of a mixture. For example it can be expressed as molar fraction, volume fraction, mass fraction, molality, molarity or normality. Chemical composition of a mixture can be represented graphically in plots like ternary plot and quaternary plot.
Mixture is a material system made up of two or more different substances which are mixed but are not combined chemically. A mixture refers to the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions, and colloids. Extraction.
Volume is the amount of 3-dimensional space occupied by an object. A relative amount.
Concentration is the strength of a solution or the number of molecules of a substance in a given volume. The spatial property of being crowded together. Increase in density or the amount per unit size.
Homogeneous is consisting of parts that are alike or all of the same kind. Something that is uniform in nature or character throughout. In the context of chemistry, homogenous is used to describe a mixture that is uniform in structure or composition. Homogeneous material or system has the same properties at every point; it is uniform without irregularities. A uniform electric field would be compatible with homogeneity.
Uniform is being the same throughout in structure or composition. Always the same or showing a single form or character in all occurrences. Not differentiated. Evenly spaced.
Reactions
Reaction is a process in which one or more substances are changed into others. A bodily process occurring due to the effect of some antecedent stimulus or agent. Reaction in mechanics is the equal and opposite force that is produced when any force is applied to a body. Reaction can also mean an idea evoked by some experience. A response that reveals a person's feelings or attitude.
Chemical Reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation.
Electrochemical Reaction - Catalysis - Thermal Reactions - Explosions (combustion) - Cause and Effect
Chemical Energy is the potential of a chemical substance to undergo a transformation through a chemical reaction to transform other chemical substances. Examples include batteries, gasoline, food and more. Breaking or making of chemical bonds involves energy, which may be either absorbed or evolved from a chemical system.
Criticality Accident is an uncontrolled nuclear fission chain reaction. It is sometimes referred to as a critical excursion, a critical power excursion, or a divergent chain reaction.
Interaction is a kind of action that occurs as two or more objects have an effect upon one another. Interface.
Bond - Force of Attraction - Coexist - Fundamental Interaction
A single-molecule guide to understanding chemical reactions better. Scientists from Tokyo Institute of Technology decided to explore DNA "hybridization" (formation of a double-stranded DNA from two single-stranded DNA) by measuring the changes in single-molecule electrical conductivity using an scanning tunneling microscope. Single-molecule investigations can often reveal new details on chemical and biological processes that cannot be identified in a bulk collection of molecules due to the averaging out of individual molecule behavior.
Inert is used to describe a substance that is not chemically reactive. From a thermodynamic perspective, a substance is inert, or nonlabile, if it is thermodynamically unstable (positive standard Gibbs free energy of formation) yet decomposes at a slow, or negligible rate. Inert Gas is a gas that does not undergo chemical reactions under a set of given conditions. The noble gases often do not react with many substances and were historically referred to as the inert gases. Inert gases are used generally to avoid unwanted chemical reactions degrading a sample. These undesirable chemical reactions are often oxidation and hydrolysis reactions with the oxygen and moisture in air. The term inert gas is context-dependent because several of the noble gases can be made to react under certain conditions. Purified argon and nitrogen gases are most commonly used as inert gases due to their high natural abundance (78.3% N2, 1% Ar in air) and low relative cost. Unlike noble gases, an inert gas is not necessarily elemental and is often a compound gas. Like the noble gases, the tendency for non-reactivity is due to the valence, the outermost electron shell, being complete in all the inert gases. This is a tendency, not a rule, as noble gases and other "inert" gases can react to form compounds. Inert is something chemically inactive and having only a limited ability to react chemically. Slow and apathetic or unable to move or resist motion.
High Reaction Rates even without Precious Metals. Non-precious metal nanoparticles could one day replace expensive catalysts for hydrogen production. However, it is often difficult to determine what reaction rates they can achieve, especially when it comes to oxide particles. This is because the particles must be attached to the electrode using a binder and conductive additives, which distort the results. With the aid of electrochemical analyses of individual particles, researchers have now succeeded in determining the activity and substance conversion of nanocatalysts made from cobalt iron oxide -- without any binders.
Catalysis in chemistry is a substance that initiates or accelerates a chemical reaction without itself being affected. It is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst, which is not consumed in the catalyzed reaction and can continue to act repeatedly. Because of this, only very small amounts of catalyst are required to alter the reaction rate in principle. Catalysis can also mean something that causes an important event to happen.
Cofactor in biochemistry is a non-protein chemical compound or metallic ion that is required for an enzyme's activity as a catalyst, a substance that increases the rate of a chemical reaction. Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized by in an area of study called enzyme kinetics.
Magic Number refers to a specific properties (such as stability) for only certain representatives among a distribution of species.
Bacterial Enzyme Enables Reactions - Drug Research
Unimolecular Reactions is where one reactant undergoes bond breaking and/or bond forming to yield different products.
Bimolecular Reactions is where two reactants collide and then undergo bond breaking and/or forming to yield different products.
Termolecular Association Reactions is where two reactants collide to form a molecular complex with a new chemical bond between the two reactants and a third molecule, known as the bath gas, removes some of the internal kinetic energy of that molecule to stabilize it. New class of chemical reactions involving three molecules that each participate in the breaking and forming of chemical bonds. The reaction of three different molecules is enabled by an "ephemeral collision complex," formed from the collision of two molecules, which lives long enough to collide with a third molecule. Cause and Effect.
Single Molecule Control for a Millionth of a Billionth of a Second.
Mechanical Force as a new way of starting Chemical Reactions. Researchers have shown mechanical force can start chemical reactions, making them cheaper, more broadly applicable, and more environmentally friendly than conventional methods.
Researchers monitor electron behavior during chemical reactions for the first using laser pulses and supercomputing simulations, researchers observe electrons' motions in real time.
Chemical Ecology examines the role of chemical interactions between living organisms and their environment, as the consequences of those interactions on the ethology and evolution of the organisms involved. It is thus a vast and highly interdisciplinary field. Chemical ecology studies focuses on the biochemistry of ecology and the specific molecules or groups of molecules termed semiochemicals that function as signals to initiate, modulate, or terminate a variety of biological processes such as metabolism. Molecules that serve in such roles typically are readily diffusible organic substances of low molecular mass that derive from secondary metabolic pathways, but also include peptides and other natural products. Chemical ecological processes mediated by semiochemicals include ones that are intraspecific (occurring within a species) or that are interspecific (occurring between species). A variety of functional subtypes of signals are known, including pheromones, allomones, kairomones, and attractants and repellents. It can sometimes be hard to differentiate from other biological fields and may require many disciplines working together in a study.
Briggs-Rauscher Reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking colour changes: the freshly prepared colourless solution slowly turns an amber colour, suddenly changing to a very dark blue. This slowly fades to colourless and the process repeats, about ten times in the most popular formulation, before ending as a dark blue liquid smelling strongly of iodine.
Mysterious organic scum boosts chemical reaction efficiency, may reduce chemical waste. Chemical manufacturers frequently use toxic solvents such as alcohols and benzene to make products like pharmaceuticals and plastics. Researchers are examining a previously overlooked and misunderstood phenomenon in the chemical reactions used to make these products. This discovery brings a new fundamental understanding of catalytic chemistry and a steppingstone to practical applications that could someday make chemical manufacturing less wasteful and more environmentally sound.
Functional Group are specific groups (moieties) of atoms or bonds within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of. This allows for systematic prediction of chemical reactions and behavior of chemical compounds and design of chemical syntheses. Furthermore, the reactivity of a functional group can be modified by other functional groups nearby. In organic synthesis, functional group interconversion is one of the basic types of transformations.
Identifying right-handed and left-handed molecules is a crucial step for many applications in chemistry and pharmaceutics. Chemists have now presented an original and very sensitive method. The researchers use laser pulses of extremely short duration to excite electrons in molecules into twisting motion, the direction of which reveals the molecules’ handedness.
Chirality is a geometric property of some molecules and ions. A chiral molecule/ion is non-superimposable on its mirror image. The presence of an asymmetric carbon center is one of several structural features that induce chirality in organic and inorganic molecules.
Absolute Configuration refers to the spatial arrangement of the atoms of a chiral molecular entity (or group) and its stereochemical description e.g. R or S, referring to Rectus, or Sinister, respectively.
Chemical Polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have a geometry which is asymmetric in at least one direction, so that the bond dipoles do not cancel each other. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Water (H2O) is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other.
Synthesis - Enzymes
Chemical Similarity refers to the similarity of chemical elements, molecules or chemical compounds with respect to either structural or functional qualities, i.e. the effect that the chemical compound has on reaction partners in inorganic or biological settings. Biological effects and thus also similarity of effects are usually quantified using the biological activity of a compound. In general terms, function can be related to the chemical activity of compounds (among others).
Chemistry Types
Organic Chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure includes many physical and chemical methods to determine the chemical composition and the chemical constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes probing their scope through use in preparation of target compounds (e.g., natural products, drugs, polymers, etc.) by chemical synthesis, as well as the focused study of the reactivities of individual organic molecules, both in the laboratory and via theoretical (in silico) study. A crash course in Organic Chemistry: Jakob Magolan (youtube).
Organic Materials Database - Green Chemistry - Medicinal Chemistry
Inorganic Chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Synthetic Biology.
Nuclear Chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.
Physical Chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of the principles, practices and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibrium.
Photo-Electro-Chemistry is a subfield of study within physical chemistry concerned with the interaction of Light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the German electrochemist Heinz Gerischer. The interest in this domain is high in the context of development of renewable energy conversion and storage technology.
Photo-Chemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400 – 750 nm) or infrared radiation (750 – 2500 nm). In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D with sunlight. Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry is also destructive, as illustrated by the photodegradation of plastics.
Photonics - Fuel Cells - Electrochemistry (Batteries)
Stereo-Chemistry is a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality".
Biochemistry sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is on understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of tissues, organs, and whole organisms-that is, all of biology. Biochemist are scientists that are trained in biochemistry.
Bioreactor - Bioenergy
Polymer Chemistry is a chemistry subdiscipline that deals with the structures, chemical synthesis and properties of polymers, primarily synthetic polymers such as plastics and elastomers. Polymer chemistry is related to the broader field of polymer science, which also encompasses polymer physics and polymer engineering. Molding.
Shape-Memory Polymer are polymeric smart materials that have the ability to return from a deformed state (temporary shape) to their original (permanent) shape induced by an external stimulus (trigger), such as temperature change. Shape-memory materials "remember" their original shape and return to it after they are deformed. They are commonly metallic alloys that make possible "unbreakable" eyeglass frames and quieter jet engines.
Natural Rubber consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds, plus water. Malaysia and Indonesia are two of the leading rubber producers. Forms of polyisoprene that are used as natural rubbers are classified as elastomers. Currently, rubber is harvested mainly in the form of the latex from the rubber tree or others. The latex is a sticky, milky colloid drawn off by making incisions in the bark and collecting the fluid in vessels in a process called "tapping". The latex then is refined into rubber ready for commercial processing. In major areas, latex is allowed to coagulate in the collection cup. The coagulated lumps are collected and processed into dry forms for marketing. Natural rubber is used extensively in many applications and products, either alone or in combination with other materials. In most of its useful forms, it has a large stretch ratio and high resilience, and is extremely waterproof.
Rubber - Ames Laboratory, UConn discover superconductor with bounce.
Polymerization is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. Polymers are high molecular mass compounds formed by polymerization of monomers, which is a molecule that, as a unit, binds chemically or supramolecularly to other molecules to form a supramolecular polymer.
Polyurethane is a polymer composed of organic units joined by carbamate (urethane) links. While most polyurethanes are thermosetting polymers that do not melt when heated, thermoplastic polyurethanes are also available.
Polyester is a category of polymers that contain the ester functional group in their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in the cutin of plant cuticles, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. The material is used extensively in clothing.
Polyethylene is the most common plastic, with around 80 million tonnes being produced annually. Its primary use is in packaging (plastic bags, plastic films, geomembranes, containers including bottles, etc.). Many kinds of polyethylene are known, with most having the chemical formula (C2H4)n. PE is usually a mixture of similar polymers of ethylene with various values of n. Polyethylene is a thermoplastic however can become a thermoset plastic when modified (such as cross-linked polyethylene).
Analytical Chemistry (Tools) - Distillation
Geo-Chemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the entire Solar System and has made important contributions to the understanding of a number of processes including mantle convection, the formation of planets and the origins of granite and basalt.
Computational Chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids. It is necessary because, apart from relatively recent results concerning the hydrogen molecular ion (dihydrogen cation, see references therein for more details), the quantum many-body problem cannot be solved analytically, much less in closed form. While computational results normally complement the information obtained by chemical experiments, it can in some cases predict hitherto unobserved chemical phenomena. It is widely used in the design of new drugs and materials.
Mechanochemistry is the coupling of mechanical and chemical phenomena on a molecular scale and includes mechanical breakage, chemical behaviour of mechanically stressed solids (e.g., stress-corrosion cracking or enhanced oxidation), tribology, polymer degradation under shear, cavitation-related phenomena (e.g., sonochemistry and sonoluminescence), shock wave chemistry and physics, and even the burgeoning field of molecular machines. Mechanochemistry can be seen as an interface between chemistry and mechanical engineering. It is possible to synthesize chemical products by using only mechanical action. The mechanisms of mechanochemical transformations are often complex and different from usual thermal or photochemical mechanisms. The method of ball milling is a widely used process in which mechanical force is used to achieve chemical processing and transformations. The special issue of Chemical Society Review (vol. 42, 2013, Issue 18) is dedicated to the theme of mechanochemistry. Fundamentals and applications ranging from nano materials to technology have been reviewed. The mechanochemical approach has been used to synthesize metallic nanoparticles, catalysts, magnets, ?-graphyne, metal iodates, nickel–vanadium carbide and molybdenum–vanadium carbide nanocomposite powders. Mechanochemistry is radically different from the traditional way of dissolving, heating and stirring chemicals in a solution. Because it eliminates the need for many solvents, mechanochemistry could help make many chemical processes used by industry more environmentally friendly.For example, the mechanochemical process has been used as an environmentally preferable way to synthesize pharmaceutically-attractive phenol hydrazones. The term mechanochemistry is sometimes confused with mechanosynthesis, which refers specifically to the machine-controlled construction of complex molecular products. Mechanochemical phenomena have been utilized since time immemorial, for example in making fire. The oldest method of making fire is to rub pieces of wood against each other, creating friction and hence heat, allowing the wood to undergo combustion at a high temperature. Another method involves the use of flint and steel, during which a spark (a small particle of pyrophoric metal) spontaneously combusts in air, starting fire instantaneously.
Theoretical Chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface of potential energy, molecular orbitals, orbital interactions, molecule activation, etc.
Analytical Chemistry studies and uses instruments and methods used to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative Analysis identifies analytes and seeks to find the elemental composition of inorganic compounds, while Quantitative Analysis determines the numerical amount or concentration and the determination of the absolute or relative abundance. Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation, which is a separation technique in which a field (thermal, electric, magnetic, hydraulic, gravitational, ...) is applied to a diluted suspension in a fluid or to a solution pumped through a long and narrow channel, perpendicular to the direction of the field, in order to cause the separation of particles present in the fluid, depending on their differing "mobilities" under the force exerted by the field. Then qualitative and quantitative analysis can be performed, often with the same instrument and may use light interaction, heat interaction, electric fields or magnetic fields. Often the same instrument can separate, identify and quantify an analyte. Analyte is a substance or chemical constituent that is of interest in an analytical procedure. Analytical chemistry is also focused on improvements in experimental design, chemometrics, and the creation of new measurement tools. Analytical chemistry has broad applications to medicine, science and engineering. Lab Work.
Analytical is using skilled analysis such as separating a whole into its elemental parts or basic principles. Analytical in logic is of a proposition that is necessarily true independent of fact or experience.
Process Analytical Chemistry is the application of analytical chemistry with specialized techniques, algorithms, and sampling equipment for solving problems related to chemical processes. It is a specialized form of analytical chemistry used for process manufacturing similar to process analytical technology (PAT) used in the pharmaceutical industry. The chemical processes are for production and quality control of manufactured products, and process analytical technology is used to determine the physical and chemical composition of the desired products during a manufacturing process.
Analytical Balance is a class of balance designed to measure small mass in the sub-milligram range. The measuring pan of an analytical balance (0.1 mg or better) is inside a transparent enclosure with doors so that dust does not collect and so any air currents in the room do not affect the balance's operation. This enclosure is often called a draft shield. The use of a mechanically vented balance safety enclosure, which has uniquely designed acrylic airfoils, allows a smooth turbulence-free airflow that prevents balance fluctuation and the measure of mass down to 1 μg without fluctuations or loss of product. Also, the sample must be at room temperature to prevent natural convection from forming air currents inside the enclosure from causing an error in reading. Single pan mechanical substitution balance maintains consistent response throughout the useful capacity is achieved by maintaining a constant load on the balance beam, thus the fulcrum, by subtracting mass on the same side of the beam to which the sample is added. Electronic analytical scales measure the force needed to counter the mass being measured rather than using actual masses. As such they must have calibration adjustments made to compensate for gravitational differences. They use an electromagnet to generate a force to counter the sample being measured and outputs the result by measuring the force needed to achieve balance. Such measurement device is called electromagnetic force restoration sensor.
Detection Limit or Limit of Detection is the lowest quantity of a substance that can be distinguished from the absence of that substance (a blank value) with a stated confidence level (generally 99%). LOD or Limit of Detection is another consideration that affects the detection limit is the accuracy of the model used to predict concentration from the raw analytical signal. Limit of Quantitation or LOQ describes the smallest concentration of a substance that can be reliably measured by an analytical procedure. Quantitation is to express something as a number, measure or quantity. To determine or measure the quantity of something.
Synthesis
Synthesis refers to a combination of two or more entities that together form something new; alternately, it refers to the creating of something by artificial means, or recreating or imitating a chemical compound or substance produced by a living organism that is natural and found in nature. The process of producing a chemical compound, usually by the union of simpler chemical compounds. Bonds - Alchemy - Extraction.
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined together to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide bonds, and DNA molecules, which are composed of nucleotides joined via phosphodiester bonds. Waste Energy.
Chemical Synthesis is a purposeful execution of chemical reactions to obtain a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions. In modern laboratory usage, this tends to imply that the process is reproducible, reliable, and established to work in multiple laboratories. A chemical synthesis begins by selection of compounds that are known as reagents or reactants. Various reaction types can be applied to these to synthesize the product, or an intermediate product. This requires mixing the compounds in a reaction vessel such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of work-up procedure before the final product is isolated. Chemical synthesis is the construction of complex chemical compounds from simpler ones. It is the process by which many substances important to daily life are obtained. Photosynthesis - Electro-Chemistry - Synthetic Biology.
Protein Biosynthesis is the process whereby biological cells generate new proteins; it is balanced by the loss of cellular proteins via degradation or export. Translation, the assembly of amino acids by ribosomes, is an essential part of the biosynthetic pathway, along with generation of messenger RNA (mRNA), aminoacylation of transfer RNA (tRNA), co-translational transport, and post-translational modification. Protein biosynthesis is strictly regulated at multiple steps. They are principally during transcription (phenomena of RNA synthesis from DNA template) and translation (phenomena of amino acid assembly from RNA). Build Blocks of Life.
Protein Synthesis is the process of how proteins are assembled from amino acids using information encoded in genes. Each protein has its own unique amino acid sequence that is specified by the nucleotide sequence of the gene encoding this protein. The genetic code is a set of three-nucleotide sets called codons and each three-nucleotide combination designates an amino acid, for example AUG (adenine-uracil-guanine) is the code for methionine. Because DNA contains four nucleotides, the total number of possible codons is 64; hence, there is some redundancy in the genetic code, with some amino acids specified by more than one codon. Simplified method makes cell-free protein synthesis more flexible and accessible.
Amino Acids
Amino Acid are biologically important organic compounds containing amine (-NH2) and carboxyl (-COOH) functional groups, along with a side-chain (R group) specific to each amino acid. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen, though other elements are found in the side-chains of certain amino acids. About 500 amino acids are known (though only 20 appear in the genetic code) and can be classified in many ways. Nine proteinogenic amino acids are called "essential" for humans because they cannot be created from other compounds by the human body and so must be taken in as food. Others may be conditionally essential for certain ages or medical conditions. Essential amino acids may also differ between species. Because of their biological significance, amino acids are important in nutrition and are commonly used in nutritional supplements, fertilizers, and food technology. Industrial uses include the production of drugs, biodegradable plastics, and chiral catalysts. Amino acids perform critical roles in processes such as neurotransmitter transport and biosynthesis.
BCAA or Branched Chain Amino Acids. Amino acids are the building blocks of protein. There are nine essential amino acids in total, but there's a key trio that helps you maintain muscle: leucine, isoleucine, and valine. Of these three, leucine is the muscle-building powerhouse. The beauty of BCAA supplements is they can be easily used during exercise to reduce fatigue, accelerate recovery, reduce muscle soreness, and improve the use of fat for energy. BCAAs are well known for triggering protein synthesis. Leucine is an α-amino acid used in the biosynthesis of proteins. Isoleucine is an α-amino acid that is used in the biosynthesis of proteins. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Valine is an α-amino acid that is used in the biosynthesis of proteins. Human dietary sources are any proteinaceous foods such as meats, dairy products, soy products, beans and legumes. Vitamins - Minerals - Vegetables.
Peptide are natural biological or artificially manufactured short chains of amino acid monomers linked by peptide (amide) bonds.
Catalysis
Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. In most cases, reactions occur faster with a catalyst because they require less activation energy. Furthermore, since they are not consumed in the catalyzed reaction, catalysts can continue to act repeatedly. Often only tiny amounts are required in principle.
Autocatalysis. A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if one of the reaction products is also a reactant and therefore a catalyst in the same or a coupled reaction. The reaction is called an autocatalytic reaction. Photo-Catalysis.
Order of Reaction in chemical kinetics, the order of reaction with respect to a given substance (such as reactant, catalyst or product) is defined as the index, or exponent, to which its concentration term in the rate equation is raised.
Enzymes
Enzyme are macromolecular biological catalysts. Enzymes accelerate chemical reactions. The molecules upon which enzymes may act are called substrates and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognizing that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. The latter are called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction rate by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many therapeutic drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH. Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew. Six types of enzymes are as follows: oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. Hydrolases are the most common type, followed by oxioreductases and transferases. They account for over half of the known enzymes.
Enzyme Inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.
Coenzyme is a small molecule essential for the activity or functioning of some enzymes. (not a protein but sometimes a vitamin). Soluble.
Cofactor is a substance that must join with another to produce a given result. (as a coenzyme).
Cofactor in biochemistry is a non-protein chemical compound or metallic ion that is required for a Protein's biological activity to happen. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which this happen are characterized by enzyme kinetics. Cofactors can be subclassified as either inorganic ions or complex organic molecules called coenzymes, the latter of which is mostly derived from vitamins and other organic essential nutrients in small amounts. A coenzyme that is tightly or even covalently bound is termed a prosthetic group. Cosubstrates are transiently bound to the protein and will be released at some point, then get back in. The prosthetic groups, on the other hand, are bound permanently to the protein. Both of them have the same function, which is to facilitate the reaction of enzymes and protein. Additionally, some sources also limit the use of the term "cofactor" to inorganic substances. An inactive enzyme without the cofactor is called an apoenzyme, while the complete enzyme with cofactor is called a holoenzyme. Some enzymes or enzyme complexes require several cofactors. For example, the multienzyme complex pyruvate dehydrogenase at the junction of glycolysis and the citric acid cycle requires five organic cofactors and one metal ion: loosely bound thiamine pyrophosphate (TPP), covalently bound lipoamide and flavin adenine dinucleotide (FAD), and the cosubstrates nicotinamide adenine dinucleotide (NAD+) and coenzyme A (CoA), and a metal ion (Mg2+). Organic cofactors are often vitamins or made from vitamins. Many contain the nucleotide adenosine monophosphate (AMP) as part of their structures, such as ATP, coenzyme A, FAD, and NAD+. This common structure may reflect a common evolutionary origin as part of ribozymes in an ancient RNA world. It has been suggested that the AMP part of the molecule can be considered to be a kind of "handle" by which the enzyme can "grasp" the coenzyme to switch it between different catalytic centers.
Cells and Longevity
Chemical Kinetics also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that can describe the characteristics of a chemical reaction.
Petrochemical are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane.
Litmus is a water soluble mixture of different dyes extracted from lichens. It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity.
PH - Water
Structural Formula of a chemical compound is a graphic representation of the molecular structure, showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike chemical formulas, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same chemical formula. A structural formula is able to indicate arrangements of atoms in three dimensional space in a way that a chemical formula may not be able to do. Also known as Representation in chemistry.
Unbalanced Equation. Chemical equations usually do not come already balanced. Therefore, we must finish our chemical reaction with as many atoms of each element as when we started. Example #1: Balance the following equation: H2 + O2 ---> H2O. It is an unbalanced equation (sometimes also called a skeleton equation). A skeleton equation is just a way of using the formulas to indicate the chemicals that were involved in the chemical reaction. "Mg + O2 MgO." This skeleton equation shows that magnesium reacts with oxygen to form magnesium oxide.
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons, primarily protons and neutrons.
Extraction
Extract is a substance made by extracting a part of a raw material, often by using a solvent such as ethanol or water. Extracts may be sold as tinctures, absolutes or in powder form. The aromatic principles of many spices, nuts, herbs, fruits, etc., and some flowers, are marketed as extracts, among the best known of true extracts being almond, cinnamon, cloves, ginger, lemon, nutmeg, orange, peppermint, pistachio, rose, spearmint, vanilla, violet, and wintergreen. Bond.
Extraction in chemistry is a separation process consisting in the separation of a substance from a matrix. It includes Liquid-liquid extraction, and Solid phase extraction. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte move from the water into an organic layer. Synthesize.
Centrifuge is a machine with a rapidly rotating container that applies centrifugal force to its contents, typically to separate fluids of different densities (e.g., cream from milk) or liquids from solids. is a piece of equipment that puts an object in rotation around a fixed axis (spins it in a circle), applying a force perpendicular to the axis of spin (outward) that can be very strong. The centrifuge works using the sedimentation principle, where the centrifugal acceleration causes denser substances and particles to move outward in the radial direction. At the same time, objects that are less dense are displaced and move to the center. In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low-density substances rise to the top.
Separation Process is a method that converts a mixture or solution of chemical substances into two or more distinct product mixtures. At least one of results of the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties (such as size, shape, mass, density, or chemical affinity) between the constituents of a mixture. Processes are often classified according to the particular differences they use to achieve separation. If no single difference can be used to accomplish a desired separation, multiple operations can often be combined to achieve the desired end. With a few exceptions, elements or compounds exist in nature in an impure state. Often these raw materials must go through a separation before they can be put to productive use, making separation techniques essential for the modern industrial economy. The purpose of a separation may be analytical, can be used as a lie components in the original mixture without any attempt to save the fractions, or may be preparative, i.e. to "prepare" fractions or samples of the components that can be saved. The separation can be done on a small scale, effectively a laboratory scale for analytical or preparative purposes, or on a large scale, effectively an industrial scale for preparative purposes, or on some intermediate scale. Filtering - Membrane.
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into smaller particles such as atoms, ions or radicals, usually in a reversible manner. For instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (H+) and a negative ion. Dissociation is the opposite of association or recombination.
Molecular Diffusion is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the end result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.
Diffusion is the net movement of molecules or atoms from a region of high concentration (or high chemical potential) to a region of low concentration (or low chemical potential). This is also referred to as the movement of a substance down a concentration gradient. A gradient is the change in the value of a quantity (e.g., concentration, pressure, temperature) with the change in another variable (usually distance). For example, a change in concentration over a distance is called a concentration gradient, a change in pressure over a distance is called a pressure gradient, and a change in temperature over a distance is a called a temperature gradient. Soluble.
Intrinsic Properties and Extrinsic Properties is a property of a system or of a material itself or within. It is independent of how much of the material is present and is independent of the form of the material, e.g., one large piece or a collection of small particles. Intrinsic properties are dependent mainly on the chemical composition or structure of the material.
Quantitative Analysis is the determination of the absolute or relative abundance (often expressed as a concentration) of one, several or all particular substance(s) present in a sample.
Receptor is a protein molecule that receives chemical signals from outside a cell. When such chemical signals bind to a receptor, they cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. In this sense, a receptor is a protein-molecule that recognizes and responds to endogenous chemical signals, e.g. an acetylcholine receptor recognizes and responds to its endogenous ligand, acetylcholine. However, sometimes in pharmacology, the term is also used to include other proteins that are drug targets, such as enzymes, transporters and ion channels.
Base Chemistry are substances that, in aqueous solution, are slippery to the touch, taste astringent, change the color of indicators (e.g., turn red litmus paper blue), react with acids to form salts, promote certain chemical reactions (base catalysis), accept protons from any proton donor, and/or contain completely or partially displaceable OH− ions. Examples of bases are the hydroxides of the alkali metals and alkaline earth metals (NaOH, Ca(OH)2, etc.).
Elements - Periodic Table
Element is any of the more than 100 known substances, of which 92 occur naturally, that cannot be separated into simpler substances and that singly or in combination constitute all matter. Each element is distinguished by its Atomic Number, which is the number of protons in the nuclei of its atoms. Element can also mean an abstract part of something. An artifact that is one of the individual parts of which a composite entity is made up; especially a part that can be separated from or attached to a system.
Chemical Element is a species of atoms having the same number of protons in their atomic nuclei. There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up Earth, while oxygen is the most common element in the Earth's crust. Chemical elements constitute all of the ordinary matter of the universe.
Elements are atoms, the smallest piece that we can split matter into, except for subatomic particles and other things. Elements often are stacked together with other elements to form minerals. Native elements that occur naturally are also considered minerals. Native Element Minerals are those elements that occur in nature in uncombined form with a distinct mineral structure. The elemental class includes metals and intermetallic elements, naturally occurring alloys, semi-metals and non-metals. The Nickel–Strunz classification system also includes the naturally occurring phosphides, silicides, nitrides and carbides.
Periodic Table is a tabular arrangement of the chemical elements, arranged by atomic number, electron configuration, and recurring chemical properties, whose structure shows periodic trends.
Periodic is something happening or recurring at regular intervals. Recurring or reappearing from time to time.
Periodic Table of Elements (image) - Earth & Sky Chart (image)
118 different Elements and more than 109 different types of atom - one for each element. Only the first 92 elements in the table are naturally found. Matter - Molecule - Minerals - Materials.
Periodic Table of Elements (interactive)
Wiki Periodic Table (wiki) - Natural Elements (wiki periodic table)
Videos of the Periodic Table - Tom Lehrer - The Elements - LIVE FILM From Copenhagen in 1967 (youtube)
Synthetic Element is a chemical element that does not occur naturally on Earth, and can only be created artificially. So far, 24 synthetic elements have been created (those with atomic numbers 95–118). All are unstable, decaying with half-lives ranging from 15.6 million years to a few hundred microseconds. Seven other elements were first created artificially and thus considered synthetic, but later discovered to exist naturally (in trace quantities) as well; among them plutonium—first synthesized in 1940—the one best known to laypeople, because of its use in atomic bombs and nuclear reactors.
Chalcogen are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. It consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po). The chemically uncharacterized synthetic element livermorium (Lv) is predicted to be a chalcogen as well. Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper (the term was also used for bronze/brass, any metal in the poetic sense, ore or coin), and the Latinised Greek word genēs, meaning born or produced.
Monatomic Gas means "single atom." It is usually applied to gases: a monatomic gas is one in which atoms are not bound to each other. All chemical elements will be monatomic in the gas phase at sufficiently high temperatures. The thermodynamic behavior of monatomic gas is extremely simple when compared to polyatomic gases because it is free of any rotational or vibrational energy.
Diatomic are molecules composed of only two atoms, of the same or different chemical elements.
Noble Gas are all odorless, colorless, monatomic gases with very low chemical reactivity. The six noble gases that occur naturally are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). Oganesson (Og) is predicted to be a noble gas as well, but its chemistry has not yet been investigated.
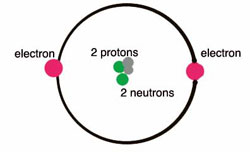 Helium is a chemical
element with symbol He and atomic number 2. It is a colorless, odorless,
tasteless, non-toxic, inert, monatomic gas, the first in the noble gas
group in the periodic table. Its boiling point is the lowest among all
the elements. After hydrogen, helium is the second lightest and second
most abundant element in the observable universe, being present at about
24% of the total elemental mass, which is more than 12 times the mass of
all the heavier elements combined. Its abundance is similar to this
figure in the Sun and in Jupiter. This is due to the very high nuclear
binding energy (per nucleon) of helium-4 with respect to the next three
elements after helium. This helium-4 binding energy also accounts for why
it is a product of both nuclear fusion and radioactive decay. Most helium
in the universe is helium-4, and is believed to have been formed during
the Big Bang. Large amounts of new helium are being created by
nuclear
fusion of hydrogen in stars.
Helium is a chemical
element with symbol He and atomic number 2. It is a colorless, odorless,
tasteless, non-toxic, inert, monatomic gas, the first in the noble gas
group in the periodic table. Its boiling point is the lowest among all
the elements. After hydrogen, helium is the second lightest and second
most abundant element in the observable universe, being present at about
24% of the total elemental mass, which is more than 12 times the mass of
all the heavier elements combined. Its abundance is similar to this
figure in the Sun and in Jupiter. This is due to the very high nuclear
binding energy (per nucleon) of helium-4 with respect to the next three
elements after helium. This helium-4 binding energy also accounts for why
it is a product of both nuclear fusion and radioactive decay. Most helium
in the universe is helium-4, and is believed to have been formed during
the Big Bang. Large amounts of new helium are being created by
nuclear
fusion of hydrogen in stars.A Tour of the Periodic Table (youtube)
Periodic Table of Elements - Chemistry: A Volatile History - BBC Four (youtube)
Investigating the Periodic Table with Experiments - with Peter Wothers (youtube)
Ununseptium Uus element 117 - PAI Polyatomic Ion
Relative Atomic Mass is a dimensionless physical quantity, the ratio of the average mass of atoms of an element (from a single given sample or source) to 1⁄12 of the mass of an atom of carbon-12 (known as the unified atomic mass unit).
Actinide series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.
Lanthanide comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare earth elements.
Electron Configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical structures).
Prayer Flag colors signifies 5 elements that are always arranged in a specific order, from left to right: blue, white, red, green, yellow. Blue represents the sky, white represents the air, red symbolizes fire, green symbolizes water, and yellow symbolizes earth. All five colors together signify balance. Traditionally, prayer flags are used to promote peace, compassion, strength, wisdom and good will, that is blown by the wind to spread into all pervading space.
Minerals
Mineral by definition, is any naturally occurring, inorganic substance, often additionally characterized by an exact crystal structure. A solid homogeneous inorganic substances occurring in nature having a definite chemical composition. Its chemical structure can be exact, or can vary within limits. Minerals and Metals in Food -
Biomineralization is the process by which living organisms produce minerals, often to harden or stiffen existing tissues. Such tissues are called mineralized tissues.
Native Metal is any metal that is found in its metallic form, either pure or as an alloy, in nature.
Alloy is a mixture of metals or a mixture of a metal and another element. Alloys are defined by a metallic bonding character.
Nonmetal is a chemical element that mostly lacks metallic attributes. Physically, nonmetals tend to be highly volatile (easily vaporized), have low elasticity, and are good insulators of heat and electricity; chemically, they tend to have high ionization energy and electronegativity values, and gain or share electrons when they react with other elements or compounds. Seventeen elements are generally classified as nonmetals; most are gases (hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypton, xenon and radon); one is a liquid (bromine), and a few are solids (carbon, phosphorus, sulfur, selenium, and iodine). Graphene.
Metalloid is any chemical element which has properties in between those of metals and nonmetals, or that has a mixture of them. There is neither a standard definition of a metalloid nor complete agreement on the elements appropriately classified as such. Despite the lack of specificity, the term remains in use in the literature of chemistry.
Rocks are a composed of one or more minerals.
Rocks - Rare Earth Minerals - Mining - Metallurgy - Alchemy - Materials
Classical Element typically refers to the concepts in ancient Greece of earth, water, air, fire, and (later) aether, which were proposed to explain the nature and complexity of all matter in terms of simpler substances. Aether classical element is the material that fills the region of the universe above the terrestrial sphere.
Atomic Spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Since unique elements have characteristic (signature) spectra, atomic spectroscopy, specifically the electromagnetic spectrum or mass spectrum, is applied for determination of elemental compositions. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is between optical and mass spectrometry. Mass spectrometry generally gives significantly better analytical performance, but is also significantly more complex. This complexity translates into higher purchase costs, higher operational costs, more operator training, and a greater number of components that can potentially fail. Because optical spectroscopy is often less expensive and has performance adequate for many tasks, it is far more common. Atomic absorption spectrometers are one of the most commonly sold and used analytical devices. When atoms are excited they emit light of certain wavelengths which correspond to different colors. The emitted light can be observed as a series of colored lines with dark spaces in between; this series of colored lines is called a line or atomic spectra. Each element produces a unique set of spectral lines.
Emission Spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify the elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.
Atomic Emission Spectroscopy is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark at a particular wavelength to determine the quantity of an element in a sample. The wavelength of the atomic spectral line in the emission spectrum gives the identity of the element while the intensity of the emitted light is proportional to the number of atoms of the element.
Atomic Absorption Spectroscopy is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state. Atomic absorption spectroscopy is based on absorption of light by free metallic ions. In analytical chemistry the technique is used for determining the concentration of a particular element (the analyte) in a sample to be analyzed. AAS can be used to determine over 70 different elements in solution, or directly in solid samples via electrothermal vaporization, and is used in pharmacology, biophysics, archaeology and toxicology research.
Molecules
Molecule is formed when two or more atoms join together chemically. All molecules are in constant motion. Molecules of a liquid have more freedom of movement than those in a solid. Molecules in a gas have the greatest degree of motion. Heat, temperature and the motion of molecules are all related. Temperature is a measure of the average kinetic energy of the molecules in a material. Nano Size - Color - Signaling Molecules (cells).
Macromolecule is a very large molecule, such as protein, commonly created by the polymerization of smaller subunits (monomers). They are typically composed of thousands of atoms or more. The most common macromolecules in biochemistry are biopolymers (nucleic acids, proteins, carbohydrates and lipids) and large non-polymeric molecules (such as lipids and macrocycles). Synthetic macromolecules include common plastics and synthetic fibers as well as experimental materials such as carbon nanotubes. The smallest molecule is the diatomic hydrogen (H2), with a bond length of 0.74 Å. Effective molecular radius is the size a molecule displays in solution.
Diatomic Molecule are molecules composed of only two atoms, of the same or different chemical elements. Binary Compound.
Heteronuclear Molecule is a molecule composed of atoms of more than one chemical element. For example, a molecule of water (H2O) is heteronuclear because it has atoms of two different elements, hydrogen (H) and oxygen (O). Similarly, a heteronuclear ion is an ion that contains atoms of more than one chemical element. For example, the carbonate ion (CO32−) is heteronuclear because it has atoms of carbon (C) and oxygen (O). The lightest heteronuclear ion is the helium hydride ion (HeH+). This is in contrast to a homonuclear ion, which contains all the same kind of atom, such as the dihydrogen cation, or atomic ions that only contain one atom such as the hydrogen anion (H−).
Biomolecule is molecule that is present in living organisms, including large macromolecules such as proteins, carbohydrates, lipids, and nucleic acids, as well as small molecules such as primary metabolites, secondary metabolites, and natural products. A more general name for this class of material is biological materials. Biomolecules are usually endogenous but may also be exogenous. For example, pharmaceutical drugs may be natural products or semisynthetic (biopharmaceuticals) or they may be totally synthetic. Compounds.
Molecular Dynamics is a computer simulation method for studying the physical movements of atoms and molecules, and is thus a type of N-body simulation. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic evolution of the system. In the most common version, the trajectories of atoms and molecules are determined by numerically solving Newton's equations of motion for a system of interacting particles, where forces between the particles and their potential energies are calculated using interatomic potentials or molecular mechanics force fields. The method was originally developed within the field of theoretical physics in the late 1950s but is applied today mostly in chemical physics, materials science and the modelling of biomolecules. Differential Equations.
Molecular Engineering is the design and testing of molecular properties, behavior and interactions in order to assemble better materials, systems, and processes for specific functions. This approach, in which observable properties of a macroscopic system are influenced by direct alteration of a molecular structure, falls into the broader category of “bottom-up” design.
Nucleotide are organic molecules that serve as the monomers, or subunits, of nucleic acids like DNA and RNA. The building blocks of nucleic acids, nucleotides are composed of a nitrogenous base, a five-carbon sugar (ribose or deoxyribose), and at least one phosphate group. Thus a nucleoside plus a phosphate group yields a nucleotide.
Molecular Biology concerns the molecular basis of biological activity between biomolecules in the various systems of a cell, including the interactions between DNA, RNA, and proteins and their biosynthesis, as well as the regulation of these interactions.
Automated Small-Molecule Synthesis
Innovative experimental scheme can create tailor-made mirror molecules. The technique can make ordinary molecules spin so fast that they lose their normal symmetry and shape and instead form mirrored versions of each other.
How molecules in cells 'find' one another and organize into structures. It's critical that the cell undergo a liquid-liquid phase separation in order for two different biological process to occur.
Powerful method probes small-Molecule Structures. Small molecules -- from naturally occurring metabolites and hormones to synthetic medicines and pesticides -- can have big effects on living things. But for scientists to understand how the molecules work and how to design beneficial ones, they need to know the precise arrangement of atoms and chemical bonds. Now researchers have found a faster, simpler and potentially more reliable way to solve the structures of small molecules. Currently, the gold standard for determining small-molecule structures is X-ray crystallography. In this technique, researchers crystallize a small molecule and then bombard the crystal with X-rays, which diffract in complex patterns that reveal the molecule's 3D structure. However, producing large, high-quality crystals is time-consuming or impossible for many compounds. Researchers wondered if they could use a form of cryoelectron microscopy to characterize small molecules. Known as microcrystal-electron diffraction (MicroED), this technique was developed 5 years ago to study protein structures. In this technique, electron beams, instead of X-rays, are diffracted from crystals, which can be much smaller than those required for X-ray crystallography.The researchers first tested MicroED on a sample of powdered progesterone, which contained thousands of nanocrystals. They rotated a single crystal and collected electron diffraction data from different angles, determining the structure of the hormone at high resolution (1 angstrom) in less than 30 minutes, compared with weeks or months for X-ray crystallography. They went on to successfully characterize 11 other natural, synthetic and pharmaceutical products, including acetaminophen, ibuprofen and several antibiotics. The researchers even identified four different molecules in a mixture by studying individual nanocrystals. Using MicroED, the researchers analyzed crystals that were a billionth of the size typically needed for X-ray crystallography. The rapid, precise method has the potential to greatly accelerate research in the fields of synthetic chemistry, natural product chemistry and drug discovery, the researchers say.
Polymers
Polymer is a large molecule, or macromolecule, composed of many repeated subunits. Because of their broad range of properties, both synthetic and natural polymers play an essential and ubiquitous role in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass relative to small molecule compounds produces unique physical properties, including toughness, viscoelasticity, and a tendency to form glasses and semicrystalline structures rather than crystals. Plastic is material consisting of any of a wide range of synthetic or semi-synthetic organic compounds that are malleable and so can be molded into solid objects.
Biopolymer are polymers produced by living organisms; in other words, they are polymeric biomolecules. Since they are polymers, biopolymers contain monomeric units that are covalently bonded to form larger structures. There are three main classes of biopolymers, classified according to the monomeric units used and the structure of the biopolymer formed: polynucleotides (RNA and DNA), which are long polymers composed of 13 or more nucleotide monomers; polypeptides, which are short polymers of amino acids; and polysaccharides, which are often linear bonded polymeric carbohydrate structures. Other examples of biopolymers include rubber, suberin, melanin and lignin.
Bio-Plastics - Material Science - Smart Polymers
Photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing. A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. 3D Printing.
Single-Nucleotide Polymorphism is a variation in a single nucleotide that occurs at a specific position in the genome, where each variation is present to some appreciable degree within a population.
Monomer is a molecule that may bind chemically or supramolecularly to other molecules to form a (supramolecular) polymer.
Supramolecular Polymers is a polymer whose monomer repeat units are held together by noncovalent bonds. Non-covalent forces that hold supramolecular polymers together include coordination, π-π interactions, and hydrogen bonding. Supramolecular polymers can have physical properties similar to plastic materials, while having better processability and better recycling and self-healing properties, thanks to their reversible transition from monomer to polymer structure.
Method to change fundamental architecture of polymers. A research team has developed methods to manipulate polymers in a way that changes their fundamental structure, paving the way for potential applications in cargo delivery and release, recyclable materials, shape-shifting soft robots, antimicrobials and more.
Monomer is a molecule that may bind chemically or supramolecularly to other molecules to form a (supramolecular) polymer.
Polymerization is any process in which relatively small molecules, called monomers, combine chemically to produce a very large chainlike or network molecule, called a polymer. The monomer molecules may be all alike, or they may represent two, three, or more different compounds.
Bonds - Attractions
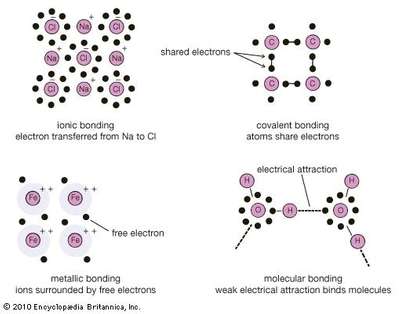 Chemical Bond is a lasting attraction between
atoms that
enables the formation of chemical compounds. The bond may result from the
electrostatic force of attraction between atoms with opposite charges, or
through the sharing of electrons as in the covalent bonds. The strength
of chemical bonds varies considerably; there are "strong bonds" or
"primary bond" such as metallic, covalent or ionic bonds and "weak bonds"
or "secondary bond" such as Dipole-dipole interaction, the London
dispersion force and hydrogen bonding.
Why form chemical bonds? One answer is that atoms are trying to
reach the most stable (lowest-energy) state that they can. Many atoms
become stable when their valence shell
is filled with electrons or when they satisfy the
octet rule by having
eight valence electrons. If atoms don’t have this arrangement, they’ll
“want” to reach it by gaining, losing, or
sharing electrons via bonds.
Chemical Bond is a lasting attraction between
atoms that
enables the formation of chemical compounds. The bond may result from the
electrostatic force of attraction between atoms with opposite charges, or
through the sharing of electrons as in the covalent bonds. The strength
of chemical bonds varies considerably; there are "strong bonds" or
"primary bond" such as metallic, covalent or ionic bonds and "weak bonds"
or "secondary bond" such as Dipole-dipole interaction, the London
dispersion force and hydrogen bonding.
Why form chemical bonds? One answer is that atoms are trying to
reach the most stable (lowest-energy) state that they can. Many atoms
become stable when their valence shell
is filled with electrons or when they satisfy the
octet rule by having
eight valence electrons. If atoms don’t have this arrangement, they’ll
“want” to reach it by gaining, losing, or
sharing electrons via bonds.Molecular Binding is an attractive interaction between two molecules that results in a stable association in which the molecules are in close proximity to each other. It often but not always involves some chemical bonding.
Bind is to fasten, attach, tie, wrap or stick things firmly together. Binding Energy - Commitment.
Bond is an electrical force linking atoms. A connection that fastens things together. The property of sticking together.
Bound in chemistry is to form a chemical bond with with another element. Bound in physics is something being held with another element, substance or material in chemical or physical union. Bound in computing is to associate an identifier with a value or object. Bound can also mean remain stuck to or to keep in place. To form the boundary of something; To be contiguous to. A line determining the limits of an area. The line or plane indicating the limit or extent of something. The greatest possible degree of something. Wrap around with something so as to cover or enclose. Fasten or secure with a rope, string, or cord. To walk or run with leaping strides. A leaping movement upward.
Ionic Bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. It is one of the main bonds along with Covalent bond and Metallic bonding. Ions are atoms that have gained one or more electrons (known as anions, which are negatively charged) and atoms that have lost one or more electrons (known as cations, which are positively charged). This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like NH+4 or SO2−4. In simpler words, an ionic bond is the transfer of electrons from a metal to a non-metal in order to obtain a full valence shell for both atoms. Zero Point Energy.
Chemical Polarity is a separation of electric charge leading to a molecule or its chemical groups having an Electric Dipole Moment, with a negatively charged end and a positively charged end. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have a geometry which is asymmetric in at least one direction, so that the bond dipoles do not cancel each other. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI units for electric dipole moment are coulomb-meter (C⋅m); however, a commonly used unit in atomic physics and chemistry is the debye (D).
Hydrogen Bond is an electrostatic attraction between two polar groups that occurs when a hydrogen (H) atom, covalently bound to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F), experiences the electrostatic field of another highly electronegative atom nearby. Hydrogen bonds can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular). Depending on the nature of the donor and acceptor atoms which constitute the bond, their geometry, and environment, the energy of a hydrogen bond can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water. and in organic molecules like DNA and proteins. Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have much weaker hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids. It also plays an important role in the structure of polymers, both synthetic and natural.
Binary Compounds of Hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Metallic Bonding is a type of chemical bonding that rises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be described as the sharing of free electrons among a structure of positively charged ions (cations). Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and luster.
Covalent Bond also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, corresponding to a stable electronic configuration. Strong bonds hold molecules together and the weaker bonds create temporary connections.
Repulsive Force is the force by which bodies repel one another. Repel is to cause something to move back or move away by force or influence. Forces.
Attractive Force is the force by which one object attracts another. There are numerous attractive forces prevailing in nature. Some of them are magnetic force, electric force, electrostatic force and gravitational force. Attract is causing something to move closer or approach, and prevent it from moving away using the force of attraction.
Dynamic Covalent Chemistry is a synthetic strategy employed by chemists to make complex supramolecular assemblies from discrete molecular building blocks. DCvC has allowed access to complex assemblies such as covalent organic frameworks, molecular knots, polymers, and novel macrocycles. Not to be confused with dynamic combinatorial chemistry, DCvC concerns only covalent bonding interactions. As such, it only encompasses a subset of supramolecular chemistries. Plastics.
Anti-Bonding Molecular Orbital is a type of molecular orbital (MO) that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more nodes in the bonding region between the nuclei. The density of the electrons in the orbital is concentrated outside the bonding region and acts to pull one nucleus away from the other and tends to cause mutual repulsion between the two atoms. This is in contrast to a bonding molecular orbital, which has a lower energy than that of the separate atoms, and is responsible for chemical bonds.
Bonding Molecular Orbital describes the attractive interactions between the atomic orbitals of two or more atoms in a molecule. In MO theory, electrons are portrayed to move in waves. When more than one of these waves come close together, the in-phase combination of these waves produces an interaction that leads to a species that is greatly stabilized. The result of the waves’ constructive interference causes the density of the electrons to be found within the binding region, creating a stable bond between the two species.
Valence is the combining power of an element, especially as measured by the number of hydrogen atoms it can displace or combine with. Valence in biology is the relative capacity to unite or react or interact as with antigens or a biological substrate.
Intermolecular Force are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between molecules and other types of neighboring particles, e.g., atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. Amazing Properties of Water.
Intramolecular Force is any force that binds together the atoms making up a molecule or compound, not to be confused with intermolecular forces, which are the forces present between molecules. The subtle difference in the name comes from the Latin roots of English with inter meaning between or among and intra meaning inside. Chemical bonds are considered to be intramolecular forces, for example. These forces are often stronger than intermolecular forces, which are present between atoms or molecules that are not bonded. Magnetism.
Binder material is any material or substance that holds or draws other materials together to form a cohesive whole mechanically, chemically, by adhesion or cohesion. In a more narrow sense, binders are liquid or dough-like substances that harden by a chemical or physical process and bind fibres, filler powder and other particles added into it. Examples include glue, adhesive and thickening. Binder Jetting is an additive manufacturing process in which a liquid binding agent is selectively deposited to join powder particles. Layers of material are then bonded to form an object. Cement.
Cohesion in chemistry is the action or property of like molecules sticking together, being mutually attractive. It is an intrinsic property of a substance that is caused by the shape and structure of its molecules, which makes the distribution of orbiting electrons irregular when molecules get close to one another, creating electrical attraction that can maintain a microscopic structure such as a water drop. In other words, cohesion allows for surface tension, creating a "solid-like" state upon which light-weight or low-density materials can be placed.
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another (cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). The forces that cause adhesion and cohesion can be divided into several types. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are also certain emergent mechanical effects. Band-Aids.
Adhesive is any non metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation. The use of adhesives offers many advantages over binding techniques such as sewing, mechanical fastening, thermal bonding, etc. These include the ability to bind different materials together, to distribute stress more efficiently across the joint, the cost effectiveness of an easily mechanized process, an improvement in aesthetic design, and increased design flexibility. Disadvantages of adhesive use include decreased stability at high temperatures, relative weakness in bonding large objects with a small bonding surface area, and greater difficulty in separating objects during testing. Adhesives are typically organized by the method of adhesion. These are then organized into reactive and non-reactive adhesives, which refers to whether the adhesive chemically reacts in order to harden. Alternatively they can be organized by whether the raw stock is of natural or synthetic origin, or by their starting physical phase. Adhesives may be found naturally or produced synthetically. The earliest human use of adhesive-like substances was approximately 200,000 years ago, when Neanderthals produced tar from the dry distillation of birch bark for use in binding stone tools to wooden handles. The first references to adhesives in literature first appeared in approximately 2000 BC. The Greeks and Romans made great contributions to the development of adhesives. In Europe, glue was not widely used until the period AD 1500–1700. From then until the 1900s increases in adhesive use and discovery were relatively gradual. Only since the last century has the development of synthetic adhesives accelerated rapidly, and innovation in the field continues to the present. Safer glues for laptops, packaging, furniture.
Dissociation is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into smaller particles such as atoms, ions or radicals, usually in a reversible manner. For instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (H+) and a negative ion. Dissociation is the opposite of recombination, which in physics is a combining of charges or transfer of electrons in a gas that results in the neutralization of ions; important for ions arising from the passage of high-energy particles.
Recombination in genetics is a combining of genes or characters different from what they were in the parents.
Heterolysis is the process of cleaving a covalent bond where one previously bonded species takes both original bonding electrons from the other species. During heterolytic bond cleavage of a neutral molecule, a cation and an anion will be generated. Most commonly the more electronegative atom keeps the pair of electrons becoming anionic while the more electropositive atom becomes cationic. Heterolytic fission almost always happens to single bonds, the process usually produces two fragment species. The energy required to break the bond is called the heterolytic bond dissociation energy, which is not equivalent to homolytic bond dissociation energy commonly used to represent the energy value of a bond.
Chirality is a geometric property of some molecules and ions. A chiral molecule/ion is non-superposable on its mirror image. The presence of an asymmetric carbon center is one of several structural features that induce chirality in organic and inorganic molecules.
Water in Space - Cells - Microbes - Organize - Metallurgy - Alchemy.
London Dispersion Force are a type of force acting between atoms and molecules. London forces are exhibited by all atoms and molecules. The electron distribution around an atom or molecule undergoes fluctuations in time. These fluctuations create instantaneous electric fields which are felt by other nearby atoms and molecules, which in turn adjust the spatial distribution of their own electrons. The net effect is that the fluctuations in electron positions in one atom induce a corresponding redistribution of electrons in other atoms, such that the electron motions become correlated. While the detailed theory requires a quantum-mechanical explanation (see quantum mechanical theory of dispersion forces), the effect is frequently described as the formation of instantaneous dipoles that (when separated by vacuum) attract each other. The magnitude of the London dispersion force is frequently described in terms of a single parameter called the Hamaker Constant, typically symbolized as "A". For atoms that are located closer together than the wavelength of light, the interaction is essentially instantaneous and is described in terms of a "non-retarded" Hamaker Constant. For entities that are farther apart, the finite time required for the fluctuation at one atom to be felt at a second atom ("retardation") requires use of a "Retarded" Hamaker constant.
Unlocking the secrets of chemical bonding with machine learning. Researchers have developed a Bayesian learning model of chemisorption, or Bayeschem for short, aiming to use artificial intelligence to unlock the nature of chemical bonding at catalyst surfaces. A new machine learning approach offers important insights into catalysis, a fundamental process that makes it possible to reduce the emission of toxic exhaust gases or produce essential materials like fabric. The d-band theory of chemisorption used in Bayeschem is a theory describing chemical bonding at solid surfaces involving d-electrons that are usually shaped like a four-leaf clover. The model explains how d-orbitals of catalyst atoms are overlapping and attracted to adsorbate valence orbitals that have a spherical or dumbbell-like shape. It has been considered the standard model in heterogeneous catalysis since its development by Hammer and Nørskov in the 1990s, and though it has been successful in explaining bonding trends of many systems, Xin said the model fails at times due to the intrinsic complexity of electronic interactions.
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbant surface. Examples include macroscopic phenomena that can be very obvious, like corrosion, and subtler effects associated with heterogeneous catalysis, where the catalyst and reactants are in different phases. The strong interaction between the adsorbate and the substrate surface creates new types of electronic bonds. In contrast with chemisorption is physisorption, which leaves the chemical species of the adsorbate and surface intact. It is conventionally accepted that the energetic threshold separating the binding energy of "physisorption" from that of "chemisorption" is about 0.5 eV per adsorbed species. Due to specificity, the nature of chemisorption can greatly differ, depending on the chemical identity and the surface structural properties. The bond between the adsorbate and adsorbent in chemisorption is either ionic or covalent.
Compound - Two Different Elements
Compound is a molecule that contains at least two different elements. Every combination of atoms is a molecule, but not all molecules are compounds. Hydrogen gas (H2) is a molecule, but not a compound because it is made of only one element. Water (H2O) can be called a molecule or a compound because it is made of hydrogen (H) and oxygen (O) atoms.
Chemical Compound is an entity consisting of two or more atoms, at least two from different chemical elements, which associate via chemical bonds. There are four types of compounds, depending on how the constituent atoms are held together: molecules held together by covalent bonds, ionic compounds held together by ionic bonds, intermetallic compounds held together by metallic bonds, and certain complexes held together by coordinate covalent bonds. Many chemical compounds have a unique numerical identifier assigned by the Chemical Abstracts Service or CAS number.
Inorganic Compound is a chemical compound where there is an absence of carbon in its composition, and is of a non-biologic origin, and cannot be found or incorporated into a living organism. Materials - Elements.
Organic Compound is virtually any chemical compound that contains carbon, although a consensus definition remains elusive and likely arbitrary. Organic compounds are rare terrestrially, but of central importance because all known life is based on organic compounds. The most basic petrochemicals are considered the building blocks of organic chemistry. There are now more than ten million Organic Compounds known by chemists.
Binary Compound is a substance composed of exactly two different elements, which are substances that cannot be simplified further by chemical means. Examples of binary compounds include H2O, H2S, and NH3. Examples of substances that are not chemical compounds include Au, Fe, O, HCN, and HNO3. Diatomic Molecule.
Binary Phase is chemical compound containing two different elements. Some binary phases compounds are molecular. More typically binary phase refers to extended solids.
Composite is a conceptual whole made up of complicated and related parts. Consisting of separate interconnected parts.
Atoms will bond together under certain environmental conditions when they are close enough. And when certain atoms bind together they can form molecules. And molecules can bind together with other molecules under certain environmental conditions. But in order for life to happen, molecules need instructions from the DNA so they will know how to bond with other molecules and know which molecules to bond with so they can create complex structures like cells that are needed to create animal life.
We don't know where atoms came from or how atoms are made or what atoms are made of. And we are not totally sure why atoms interact with other atoms. And we are not sure how atoms create molecules and not just create bigger atoms. We still don't know why exactly how electricity works, we only know that electricity exists and we only know how to control electricity to a certain degree.
Carbon
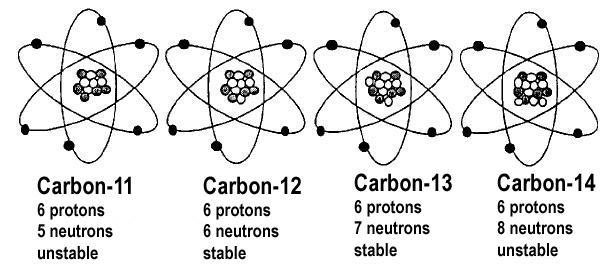 Carbon Atoms make up almost 85% of all known compounds.
Carbon Atoms can make around 1.7 million different
compounds. Carbon can form four covalent bonds to
create an organic molecule. The simplest carbon molecule is
methane or CH4.
Carbon Atoms make up almost 85% of all known compounds.
Carbon Atoms can make around 1.7 million different
compounds. Carbon can form four covalent bonds to
create an organic molecule. The simplest carbon molecule is
methane or CH4.Carbon-12 is the more abundant carbon of the two stable isotopes, amounting to 98.93% of the element carbon; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured: its mass number is 12 by definition and contains 6 protons, 6 neutrons and 6 electrons. Oxygen Atom 8-8-8 and Nitrogen is 7-7-7.
Carbon is a chemical element with symbol C and atomic number 6. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radioactive isotope, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.
Isotopes of Carbon, Carbon (6C) has 15 known isotopes, from 8C to 22C, of which 12C and 13C are stable.
Isotope are variants of a particular chemical element which differ in neutron number. All Isotopes of a given element have the same number of protons in each atom.
Carbon-Based Life is a key component of all known life on Earth. Complex molecules are made up of carbon bonded with other elements, especially oxygen, hydrogen and nitrogen, and carbon can bond with all of these because of its four valence electrons. Carbon is abundant on Earth. It is also lightweight and relatively small in size, making it easier for enzymes to manipulate carbon molecules. It is assumed in astrobiology that if life exists somewhere else in the universe, it will also be carbon-based.
Organic Compound - Organic Matter - Carbon Capture - Carbon-Fiber - Graphene
Building Blocks of Life - Human Body Composition
Pentadecane is an alkane hydrocarbon with the chemical formula C15H32.
Hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons from which one hydrogen atom has been removed are functional groups called hydrocarbyls. Because carbon has 4 electrons in its outermost shell (and because each covalent bond requires a donation of 1 electron, per atom, to the bond) carbon has exactly four bonds to make, and is only stable if all 4 of these bonds are used. Aromatic hydrocarbons (arenes), alkanes, cycloalkanes and alkyne-based compounds are different types of hydrocarbons. Most Hydrocarbons found on Earth naturally occur in crude oil, where decomposed organic matter provides an abundance of carbon and hydrogen which, when bonded, can catenate to form seemingly limitless chains. HFC's.
Thermodynamics
Thermodynamics is the branch of physics which deals with the energy and work of a system. Thermodynamics is the branch of science concerned with heat and temperature and their relation to energy and work. It states that the behavior of these quantities is governed by the four laws of thermodynamics (isobaric, isochoric, isothermal and adiabatic), irrespective of the composition or specific properties of the material or system in question. The laws of thermodynamics are explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, chemical engineering and mechanical engineering. Thermoacoustics (refrigeration).
Laws of Thermodynamics define fundamental physical quantities (temperature, energy, and entropy) that characterize thermodynamic systems at thermal equilibrium. The laws describe how these quantities behave under various circumstances, and forbid certain phenomena (such as perpetual motion).
The Second Law of Thermodynamics "what goes up must come down". Thermal Electric Energy.
Second Law of Thermodynamics dictates that everything ages, Death, and decays, and states that the total entropy of an isolated system always increases over time, or remains constant in ideal cases where the system is in a steady state or undergoing a reversible process. The increase in entropy accounts for the irreversibility of natural processes, and the asymmetry between future and past. Historically, the second law was an empirical finding that was accepted as an axiom of thermodynamic theory. Statistical thermodynamics, classical or quantum, explains the microscopic origin of the law. The second law has been expressed in many ways. There is an upper limit to the efficiency of conversion of heat to work in a heat engine.
Thermodynamic Law is a branch of science concerned with Heat and temperature and their relation to energy and work.
Isothermal Process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir (heat bath), and the change in the system will occur slowly enough to allow the system to continue to adjust to the temperature of the reservoir through heat exchange. In contrast, an adiabatic process is where a system exchanges no heat with its surroundings (Q = 0). In other words, in an isothermal process, the value ΔT = 0 and therefore the change in internal energy ΔU = 0 (only for an ideal gas) but Q ≠ 0, while in an adiabatic process, ΔT ≠ 0 but Q = 0.
Isochoric Process is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container: The thermodynamic process is the addition or removal of heat; the isolation of the contents of the container establishes the closed system; and the inability of the container to deform imposes the constant-volume condition. The isochoric process here should be a quasi-static process.
Endothermic Process is any process which requires or absorbs energy from its surroundings, usually in the form of heat. It may be a chemical process, such as dissolving ammonium nitrate in water, or a physical process, such as the melting of ice cubes.
Explosions - Combustion
Exothermic Process describes a process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen). Its etymology stems from the Greek prefix έξω (exō, which means "outwards") and the Greek word θερμικός (thermikόs, which means "thermal"). The term exothermic was first coined by Marcellin Berthelot. The opposite of an exothermic process is an endothermic process, one that absorbs energy usually in the form of heat. The concept is frequently applied in the physical sciences to chemical reactions where chemical bond energy is converted to thermal energy (heat). Exothermic (and endothermic) describe two types of chemical reactions or systems found in nature, as follows. Simply stated, after an exothermic reaction, more energy has been released to the surroundings than was absorbed to initiate and maintain the reaction. An example would be the burning of a candle, wherein the sum of calories produced by combustion (found by looking at radiant heating of the surroundings and visible light produced, including the increase in temperature of the fuel (wax) itself, which oxygen converts to hot CO2 and water vapor) exceeds the number of calories absorbed initially in lighting the flame and in the flame maintaining itself (some energy is reabsorbed and used in melting, then vaporizing the wax, etc. but is far outstripped by the energy released in converting the relatively weak double bond of oxygen to the stronger bonds in CO2 and H2O). On the other hand, in an endothermic reaction or system, energy is taken from the surroundings in the course of the reaction, usually driven by a favorable entropy increase in the system. An example of an endothermic reaction is a first aid cold pack, in which the reaction of two chemicals, or dissolving of one in another, requires calories from the surroundings, and the reaction cools the pouch and surroundings by absorbing heat from them. The production of wood by photosynthesis is an endothermic process: trees absorb radiant energy from the sun and use it in endothermic reactions such as taking apart CO2 and H2O and recombining the atoms to produce cellulose and other organic chemicals, as well as O2. The wood may later be burned in a fireplace, exothermically releasing the energy of O2 in the form of heat and light to their surroundings, e.g. to a home's interior.
Adiabatic Process occurs without transfer of heat or mass of substances between a thermodynamic system and its surroundings. In an adiabatic process, energy is transferred to the surroundings only as work. The adiabatic process provides a rigorous conceptual basis for the theory used to expound the first law of thermodynamics, and as such it is a key concept in thermodynamics. Some chemical and physical processes occur so rapidly that they may be conveniently described by the term "adiabatic approximation", meaning that there is not enough time for the transfer of energy as heat to take place to or from the system. By way of example, the adiabatic flame temperature is an idealization that uses the "adiabatic approximation" so as to provide an upper limit calculation of temperatures produced by combustion of a fuel. The adiabatic flame temperature is the temperature that would be achieved by a flame if the process of combustion took place in the absence of heat loss to the surroundings. In meteorology and oceanography, the adiabatic cooling process produces condensation of moisture or salinity and the parcel becomes oversaturated. Therefore, it is necessary to take away the excess. There the process becomes a pseudo-adiabatic process in which the liquid water/salt that condenses is assumed to be removed as soon as it is formed, by idealized instantaneous precipitation. The pseudoadiabatic process is only defined for expansion, since a parcel that is compressed become warmer and remains undersaturated.
Thermodynamic Equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium there are no net macroscopic flows of matter or of energy, either within a system or between systems. In a system in its own state of internal thermodynamic equilibrium, no macroscopic change occurs. Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, though not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, until disturbed by a thermodynamic operation. In a macroscopic equilibrium, almost or perfectly exactly balanced microscopic exchanges occur; this is the physical explanation of the notion of macroscopic equilibrium. A thermodynamic system in a state of internal thermodynamic equilibrium has a spatially uniform temperature. Its intensive properties, other than temperature, may be driven to spatial inhomogeneity by an unchanging long-range force field imposed on it by its surroundings. In systems that are at a state of non-equilibrium there are, by contrast, net flows of matter or energy. If such changes can be triggered to occur in a system in which they are not already occurring, the system is said to be in a meta-stable equilibrium. Though not a widely named a "law," it is an axiom of thermodynamics that there exist states of thermodynamic equilibrium. The second law of thermodynamics states that when a body of material starts from an equilibrium state, in which, portions of it are held at different states by more or less permeable or impermeable partitions, and a thermodynamic operation removes or makes the partitions more permeable and it is isolated, then it spontaneously reaches its own, new state of internal thermodynamic equilibrium, and this is accompanied by an increase in the sum of the entropies of the portions.
Non-Equilibrium Thermodynamics deals with physical systems that are not in thermodynamic equilibrium but can adequately be described in terms of variables (non-equilibrium state variables) that represent an extrapolation of the variables used to specify the system in thermodynamic equilibrium. Non-equilibrium thermodynamics is concerned with transport processes and with the rates of chemical reactions. It relies on what may be thought of as more or less nearness to thermodynamic equilibrium. Non-equilibrium thermodynamics is a work in progress, not an established edifice. This article will try to sketch some approaches to it and some concepts important for it. Almost all systems found in nature are not in thermodynamic equilibrium; for they are changing or can be triggered to change over time, and are continuously and discontinuously subject to flux of matter and energy to and from other systems and to chemical reactions. Some systems and processes are, however, in a useful sense, near enough to thermodynamic equilibrium to allow description with useful accuracy by currently known non-equilibrium thermodynamics. Nevertheless, many natural systems and processes will always remain far beyond the scope of non-equilibrium thermodynamic methods. This is because of the very small size of atoms, as compared with macroscopic systems.
Thermodynamic System is the material and radiative content of a macroscopic volume in space, that can be adequately described by thermodynamic state variables such as temperature, entropy, internal energy and pressure.
Chemical Thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics.
Thermal Decomposition is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion.
Thermal Expansion is the tendency of matter to change its shape, area, and volume in response to a change in temperature. Temperature is a Monotonic Function of the average molecular kinetic energy of a substance. When a substance is heated, the kinetic energy of its molecules increases. Thus, the molecules begin vibrating/moving more and usually maintain a greater average separation. Materials which contract with increasing temperature are unusual; this effect is limited in size, and only occurs within limited temperature ranges (see examples below). The relative expansion (also called strain) divided by the change in temperature is called the material's coefficient of thermal expansion and generally varies with temperature. Hot Air.
Helmholtz Free Energy is a Thermodynamic Potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature and volume. The negative of the change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which volume is held constant. If the volume were not held constant, part of this work would be performed as boundary work. This makes the Helmholtz energy useful for systems held at constant volume. Furthermore, at constant temperature, the Helmholtz energy is minimized at equilibrium.
Internal Energy of a system is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the potential energy of the system as a whole due to external force fields. It keeps account of the gains and losses of energy of the system that are due to changes in its internal state. The internal energy of a system can be changed by transfers of matter or heat or by doing work. When matter transfer is prevented by impermeable containing walls, the system is said to be closed. Then the first law of thermodynamics states that the increase in internal energy is equal to the total heat added plus the work done on the system by its surroundings. If the containing walls pass neither matter nor energy, the system is said to be isolated and its internal energy cannot change. The first law of thermodynamics may be regarded as establishing the existence of the internal energy. The internal energy is one of the two cardinal state functions of the state variables of a thermodynamic system.
Enthalpy is a property of a thermodynamic system. The enthalpy of a system is equal to the system's internal energy plus the product of its pressure and volume. For processes at constant pressure, the heat absorbed or released equals the change in enthalpy. The unit of measurement for enthalpy in the International System of Units (SI) is the joule. Other historical conventional units still in use include the British thermal unit (BTU) and the calorie. Enthalpy comprises a system's internal energy, which is the energy required to create the system, plus the amount of work required to make room for it by displacing its environment and establishing its volume and pressure. Enthalpy is defined as a state function that depends only on the prevailing equilibrium state identified by the system's internal energy, pressure, and volume. It is an extensive quantity. Enthalpy is the preferred expression of system energy changes in many chemical, biological, and physical measurements at constant pressure, because it simplifies the description of energy transfer. At constant pressure, the enthalpy change equals the energy transferred from the environment through heating or work other than expansion work. The total enthalpy, H, of a system cannot be measured directly. The same situation exists in classical mechanics: only a change or difference in energy carries physical meaning. Enthalpy itself is a thermodynamic potential, so in order to measure the enthalpy of a system, we must refer to a defined reference point; therefore what we measure is the change in enthalpy, ΔH. The ΔH is a positive change in endothermic reactions, and negative in heat-releasing exothermic processes.
Engineering Thermodynamics (Book - Wiki)
Thermoplastic
Urea is an organic compound with the chemical formula CO(NH2)2. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals, and is the main nitrogen-containing substance in the urine of mammals.
Thermodynamics of Computation (pdf)
Chemical Process Modeling is a computer modeling technique used in chemical engineering process design. It typically involves using purpose-built software to define a system of interconnected components, which are then solved so that the steady-state or dynamic behavior of the system can be predicted. The system components and connections are represented as a Process Flow diagram. Simulations can be as simple as the mixing of two substances in a tank, or as complex as an entire alumina refinery.
Triple Point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. Compound Chem.
Thermal Energy (geothermal) - Thermal Electric Energy - Solar Thermal Energy (sun energy)
Thermal Energy is the total energy an object has due to the internal motions of its particles. The temperature is related to the average kinetic energy—not the total kinetic energy. Put a piece of cold pizza on top of a sheet of aluminum foil and then stick it in the oven to heat up. After about 10 minutes, the pizza should be nice and hot—the aluminum foil is the approximately the same temperature. You can pull the aluminum foil out with your fingers, but not the pizza. Although the aluminum foil has a high temperature, its low mass means it doesn't have much thermal energy. Without a lot of thermal energy in the foil, your fingers won't get burned. Meaning? Thermal energy and temperature are different things.
Thermal Conductivity of a material is a measure of its ability to conduct heat. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. Superconductivity.
Thermal is a column of rising air in the lower altitudes of Earth's atmosphere. Thermals are created by the uneven heating of Earth's surface from solar radiation, and are an example of convection, specifically atmospheric convection. The Sun warms the ground, which in turn warms the air directly above it. Dark earth, urban areas, and roadways are good sources of thermals.
Temperature - Cold - Hot
Temperature is an objective comparative measurement of Hot or Cold. It is measured by a thermometer. Several scales and units exist for measuring temperature, the most common being Celsius (denoted °C; formerly called centigrade), Fahrenheit (denoted °F), and, especially in science, Kelvin (denoted K). The coldest theoretical temperature is absolute zero, at which the thermal motion of atoms and molecules reaches its minimum – classically, this would be a state of motionlessness, but quantum uncertainty dictates that the particles still possess a finite zero-point energy. Absolute zero is denoted as 0 K on the Kelvin scale, −273.15 °C on the Celsius scale, and −459.67 °F on the Fahrenheit scale. The kinetic theory offers a valuable but limited account of the behavior of the materials of macroscopic bodies, especially of fluids. It indicates the absolute temperature as proportional to the average kinetic energy of the random microscopic motions of those of their constituent microscopic particles, such as electrons, atoms, and molecules, that move freely within the material. Thermal vibration of a segment of protein alpha helix: The amplitude of the vibrations increases with temperature. Temperature is important in all fields of natural science including physics, geology, chemistry, atmospheric sciences, medicine and biology as well as most aspects of daily life.
Thermometer is a device that measures temperature or a temperature gradient. A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a mercury-in-glass thermometer or the pyrometric sensor in an infrared thermometer) in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value (e.g. the visible scale that is marked on a mercury-in-glass thermometer or the digital readout on an infrared model). Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research. Some of the principles of the thermometer were known to Greek philosophers of two thousand years ago. The modern thermometer gradually evolved from the thermoscope with the addition of a scale in the early 17th century and standardisation through the 17th and 18th centuries. Infrared Thermometers.
Bimetallic Strip is used to convert a temperature change into mechanical displacement. The strip consists of two strips of different metals which expand at different rates as they are heated, usually steel and copper, or in some cases steel and brass. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled. Bimetallic strip is a temperature-sensitive electrical contact used in some thermostats, consisting of two bands of different metals joined face to face along their lengths. When heated, the metals expand at different rates, causing the strip to bend. Electrical-resistance thermometers characteristically use platinum and, like thermistors, operate on the principle that electrical resistance varies with changes in temperature. However, they can measure a much greater temperature range than thermistors. Thermocouples are among the most widely used industrial thermometers. They are composed of two wires made of different materials joined together at one end and connected to a voltage-measuring device at the other. A temperature difference between the two ends creates a voltage that can be measured and translated into a measure of the temperature of the junction end. The bimetallic strip constitutes one of the most trouble-free and durable thermometers. It is simply two strips of different metals bonded together and held at one end. When heated, the two strips expand at different rates, resulting in a bending effect that is used to measure the temperature change. Thermostats formerly used bimetallic strips as temperature sensors, but modern digital thermostats use thermistors, which is a type of resistor whose resistance is strongly dependent on temperature, more so than in standard resistors.
Temperature Measurement or thermometry, describes the process of measuring a current local temperature for immediate or later evaluation. Datasets consisting of repeated standardized measurements can be used to assess temperature trends.
Thermopile is an electronic device that converts thermal energy into electrical energy. It is composed of several thermocouples connected usually in series or, less commonly, in parallel. Such a device works on the principle of the thermoelectric effect, i.e., generating a voltage when its dissimilar metals (thermocouples) are exposed to a temperature difference.
Temperature Gradient is a physical quantity that describes in which direction and at what rate the temperature changes the most rapidly around a particular location. The temperature gradient is a dimensional quantity expressed in units of degrees (on a particular temperature scale) per unit length. The SI unit is kelvin per second (K/s). It can be found in the formula for dQ/dt, the rate of heat transfer per second. Temperature gradients in the atmosphere are important in the atmospheric sciences (meteorology, climatology and related fields). T.g is defined as ratio of the difference between temperature of 2 thermometer to distance between them.
Degree in temperature is used in several scales of temperature. The symbol ° is usually used, followed by the initial letter of the unit, for example “°C” for degree(s) Celsius. A degree can be defined as a set change in temperature measured against a given scale, for example, one degree Celsius is one hundredth of the temperature change between the point at which water starts to change state from solid to liquid state and the point at which it starts to change from its gaseous state to liquid.
Room Temperature is the range of air temperatures that people prefer for indoor settings, which feel comfortable when wearing typical indoor clothing. As a medical definition, the range generally considered to be suitable for human occupancy is between 15 degrees Celsius (59 degrees Fahrenheit) and 25 °C (77 °F), though human comfort can extend somewhat beyond this range depending on factors such as humidity and air circulation. In certain fields, like science and engineering, and within a particular context, "room temperature" can have varying agreed upon values for temperature.
Celsius scale, the zero value is at the freezing point of water and the 100 value is at the Boiling Point, which depends on atmospheric conditions.
Fahrenheit, water freezes into ice is defined as 32 °F, and the boiling point of water is defined to be 212 °F, a 180 °F separation, as defined at sea level and standard atmospheric pressure. Humidity - Dew Point.
Celsius to Fahrenheit is (C times 2 plus 30 = F) Cx2+30=F
Fahrenheit to Celsius is (F minus 30 divided by 2 = C) F-30/2=C
Note: conversion numbers will not be exact, it's just to get you close to an estimate.
(0C = 32F, 27C = 80F, for every 10 Degrees F increase is equal to around a 5C increase).
Cold is the presence of low temperature, especially in the atmosphere. In common usage, cold is often a subjective perception. A lower bound to temperature is absolute zero, defined as 0.00 K on the Kelvin scale, an absolute thermodynamic temperature scale. This corresponds to −273.15 °C on the Celsius scale, −459.67 °F on the Fahrenheit scale, and 0.00 °R on the Rankine scale. Since temperature relates to the thermal energy held by an object or a sample of matter, which is the kinetic energy of the random motion of the particle constituents of matter, an object will have less thermal energy when it is colder and more when it is hotter. If it were possible to cool a system to absolute zero, all motion of the particles in a sample of matter would cease and they would be at complete rest in this classical sense. The object would be described as having zero thermal energy. Microscopically in the description of quantum mechanics, however, matter still has zero-point energy even at absolute zero, because of the uncertainty principle. Ice (water) - Ice Therapy.
Cryogenics is the production and behaviour of materials at very low temperatures. A person who studies elements that have been subjected to extremely cold temperatures is called a cryogenicist. It is not well-defined at what point on the temperature scale refrigeration ends and cryogenics begins, but scientists assume a gas to be cryogenic if it can be liquefied at or below −150 °C (123 K; −238 °F). The U.S. National Institute of Standards and Technology has chosen to consider the field of cryogenics as that involving temperatures below −180 °C (93 K; −292 °F). This is a logical dividing line, since the normal boiling points of the so-called permanent gases (such as helium, hydrogen, neon, nitrogen, oxygen, and normal air) lie below −180 °C while the Freon refrigerants, hydrocarbons, and other common refrigerants have boiling points above −180 °C. Discovery of superconducting materials with critical temperatures significantly above the boiling point of liquid nitrogen has provided new interest in reliable, low cost methods of producing high temperature cryogenic refrigeration. The term "high temperature cryogenic" describes temperatures ranging from above the boiling point of liquid nitrogen, −195.79 °C (77.36 K; −320.42 °F), up to −50 °C (223 K; −58 °F), the generally defined upper limit of study referred to as cryogenics.
Liquid Nitrogen is nitrogen in a liquid state at an extremely low temperature. It is a colorless clear liquid with a density of 0.807 g/ml at its boiling point (−195.79 °C (77 K; −320 °F)) and a dielectric constant of 1.43.
Absolute Zero is the lower limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reaches its minimum value, taken as 0. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15° on the Celsius scale (International System of Units), which equates to −459.67° on the Fahrenheit scale (United States customary units or Imperial units). The corresponding Kelvin and Rankine temperature scales set their zero points at absolute zero by definition. Ice.
Kelvin is a unit of measure for temperature based upon an absolute scale. It is one of the seven base units in the International System of Units (SI) and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all thermal motion ceases in the classical description of thermodynamics.
Quantum Super Computer is cooled to 0.01 Kelvin, which is the coldest place in the known universe, 100 times colder the space, which is 2.7 kelvins (K) (−270.45 °C; −454.81 °F).
Planck Temperature denoted by TP, is the unit of temperature in the system of natural units known as Planck units. It serves as the defining unit of the Planck temperature scale. In this scale the magnitude of the Planck temperature is equal to 1, while that of absolute zero is 0. Other temperatures can be converted to Planck temperature units. For example, 0 °C = 273.15 K = 1.9279 × 10−30TP. In SI units, the Planck temperature is about 1.417×1032 kelvin (equivalently, degrees Celsius, since the difference is trivially small at this scale), or 2.55×1032 degrees Fahrenheit or Rankine.
Planck units are a set of units of measurement defined exclusively in terms of five universal physical constants, in such a manner that these five physical constants take on the numerical value of 1 when expressed in terms of these units.
Body Temperature - Dark Matter
Spectroscopic Thermometer for Nanomaterials. A scientific team has found a new way to take the local temperature of a material from an area about a billionth of a meter wide, or approximately 100,000 times thinner than a human hair. This discovery promises to improve the understanding of useful yet unusual physical and chemical behaviors that arise in materials and structures at the nano-scale.
Entropy - Running Out of Useful Energy
Entropy is a thermodynamic quantity representing the amount of energy in a system that is no longer available for doing mechanical work. Entropy (pdf).
High Entropy would indicate less energy available for useful work in a system
Low Entropy would suggest greater energy. "entropy increases as matter and energy in the universe degrade to an ultimate state of inert uniformity".
Decay Half-Life - Cell Death - Depreciation - Dormancy - Decompose - Hibernation
Things must change in Life. If things didn't change, there would be no life. Our job as humans is to understand these changes and react to them accordingly. If things never cooled down, life could never exist. If things did not decay, then new things could not be born. Entropy is on a much slower time scale of change. A Cycle of order to disorder then back to order then to disorder again and so on and so on. Things combine and then things fall apart or decay and then they combine again and then fall apart again, and so on and so on. And when a galaxy stops making new stars, then that galaxy will eventfully fall into disorder until another galaxy can use its matter to create new stars and make new planets. And when the universe stops making new galaxies, which has not happened yet, then who knows what will happen next after that. Most likely some form of reconfiguration, which would be in trillions and trillions of years from now. So we have lots of time to think about it. But we have less time to think about our immediate threats. So its kind of stupid to think about things that will happen in trillions of years from now if we can't even live another thousand years. Death and the Cycle of Life - Carbon Dating.
Ephemeral is something lasting for a very short time. Heat.
Irreversible Process is a process that is not reversible. In thermodynamics, a change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. A system that undergoes an irreversible process may still be capable of returning to its initial state. However, the impossibility occurs in restoring the environment to its own initial conditions. An irreversible process increases the entropy of the universe. Because entropy is a state function, the change in entropy of the system is the same, whether the process is reversible or irreversible. The second law of thermodynamics can be used to determine whether a process is reversible or not. Some things look the same whether they're moving forward or moving backwards in reverse.
Entropy in statistical thermodynamics, entropy (usual symbol S) is a measure of the number of microscopic configurations Ω that correspond to a thermodynamic system in a state specified by certain macroscopic variables. Specifically, assuming that each of the microscopic configurations is equally probable, the entropy of the system is the natural logarithm of that number of configurations, multiplied by the Boltzmann constant kB (which provides consistency with the original thermodynamic concept of entropy discussed below, and gives entropy the dimension of energy divided by temperature).
Entropy and Life (pdf) - Entropy and Life (wiki)
Thermodynamic free energy is the amount of work that a thermodynamic system can perform.
Entropy in order disorder is associated with the amount of order, disorder, or chaos in a thermodynamic system.
Entropy is the number of ways we can rearrange the constituents of a system so that you don't notice. A low entropy configuration is one in which there's only a few arrangements that look that way. A high entropy arrangement is one that there are many arrangements that look that way. The second law of thermodynamics -- the law that says that entropy increases in the universe, or in some isolated bit of the universe. The reason why entropy increases is simply because there are many more ways to be high entropy than to be low entropy. Boltzmann explained that if you start with low entropy, it's very natural for it to increase because there's more ways to be high entropy. What he didn't explain was why the entropy was ever low in the first place.
Entropy in information theory systems are modeled by a transmitter, channel, and receiver. The transmitter produces messages that are sent through the channel. The channel modifies the message in some way. The receiver attempts to infer which message was sent. - PDF.
Entropic Force is a force resulting from the entire system's thermodynamical tendency to increase its entropy, rather than from a particular underlying microscopic force. For instance, the internal energy of an ideal gas depends only on its temperature, and not on the volume of its containing box, so it is not an energy effect that tends to increase the volume of the box as gas pressure does. This implies that the pressure of an ideal gas has an entropic origin. What is the origin of such an entropic force? The most general answer is that the effect of thermal fluctuations tends to bring a thermodynamic system toward a macroscopic state that corresponds to a maximum in the number of microscopic states (or micro-states) that are compatible with this macroscopic state. In other words, thermal fluctuations tend to bring a system toward its macroscopic state of maximum entropy.
Enthalpy is a measurement of energy in a thermodynamic system.
Heat Death of the Universe is a plausible ultimate fate of the universe in which the universe has evolved to a state of no thermodynamic free energy and therefore can no longer sustain processes that increase entropy. Heat death does not imply any particular absolute temperature; it only requires that temperature differences or other processes may no longer be exploited to perform work. In the language of physics, this is when the universe reaches thermodynamic equilibrium (maximum entropy). If the topology of the universe is open or flat, or if dark energy is a positive cosmological constant (both of which are consistent with current data), the universe will continue expanding forever, and a heat death is expected to occur, with the universe cooling to approach equilibrium at a very low temperature after a very long time period.
Thermal Decomposition is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion.
Biology (entropy) - Aging
Landauer's Principle is a physical principle pertaining to the lower theoretical limit of energy consumption of computation. It holds that "any logically irreversible manipulation of information, such as the erasure of a bit or the merging of two computation paths, must be accompanied by a corresponding entropy increase in non-information-bearing degrees of freedom of the information-processing apparatus or its environment. Another way of phrasing Landauer's principle is that if an observer loses information about a physical system, the observer loses the ability to extract work from that system. A so-called logically-reversible computation, in which no information is erased, may in principle be carried out without releasing any heat. This has led to considerable interest in the study of reversible computing. Indeed, without reversible computing, increases in the number of computations-per-joule-of-energy-dissipated must come to a halt by about 2050: because the limit implied by Landauer's principle will be reached by then, according to Koomey's law. At 20 °C (room temperature, or 293.15 K), the Landauer limit represents an energy of approximately 0.0172 eV, or 2.75 zJ. Theoretically, room‑temperature computer memory operating at the Landauer limit could be changed at a rate of one billion bits per second with energy being converted to heat in the memory media at the rate of only 2.85 trillionths of a watt (that is, at a rate of only 2.85 pJ/s). Modern computers use millions of times as much energy per second. Knowledge Preservation.
Limits of Computation states that there are several physical and practical limits to the amount of computation or data storage that can be performed with a given amount of mass, volume, or energy.
Bekenstein Bound is an upper limit on the entropy S, or information I, that can be contained within a given finite region of space which has a finite amount of energy—or conversely, the maximal amount of information required to perfectly describe a given physical system down to the quantum level. It implies that the information of a physical system, or the information necessary to perfectly describe that system, must be finite if the region of space and the energy is finite. In computer science, this implies that there is a maximal information-processing rate (Bremermann's limit) for a physical system that has a finite size and energy, and that a Turing machine with finite physical dimensions and unbounded memory is not physically possible.
Sun shines for billions of years and the earths core stays hot for billions of years. Humans are born with all the energy producing capabilities they need. But humans were not born with all the knowledge they need that would explain to us how to utilize energy effectively and efficiently. This is why education needs to improve. If we don't start educating people on how to accurately understand themselves and the world around them, then people will continue to make bad choices and be forced to make bad decisions, all because they don't have the necessary knowledge and information that would allow them to see life more clearly. Every human has the potential to be intelligent, but we can not utilize this potential if we never learn how. Human education is our endless supply of energy. And we will never be able to fully utilize our suns and our planets constant flow of energy if we don't improve education. Everything will be wasted and it will be a wasted opportunity. Our window of opportunity is upon us, and this window will not stay open for long. Conservation of Mass.
Chemistry Tools - Chemistry Equipment
Laboratory Equipment Names (image) - Chemistry Lab Tools Image (photo)
Chemistry Sets - Chemistry Kits - Chemistry Set History (wiki) - Science Tools - Engineering Tools
Do It Yourself Science (DIY Medicine) - DIY Biology (ethics) - Procedures
Centrifuge is a piece of equipment that puts an object in rotation around a fixed axis (spins it in a circle), applying a potentially strong force perpendicular to the axis of spin (outward). The centrifuge works using the sedimentation principle, where the centripetal acceleration causes denser substances and particles to move outward in the radial direction. At the same time, objects that are less dense are displaced and move to the center. In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low-density substances rise to the top. There are 3 types of centrifuge designed for different applications. Industrial scale centrifuges are commonly used in manufacturing and waste processing to sediment suspended solids, or to separate immiscible liquids. An example is the cream separator found in dairies. Very high speed centrifuges and ultracentrifuges able to provide very high accelerations can separate fine particles down to the nano-scale, and molecules of different masses. Large centrifuges are used to simulate high gravity or acceleration environments (for example, high-G training for test pilots). Medium-sized centrifuges are used in washing machines and at some swimming pools to wring water out of fabrics. Gas centrifuges are used for isotope separation, such as to enrich nuclear fuel for fissile isotopes.
Stanford Bioengineers Develop a 20-cent, Hand-Powered Centrifuge "Hand-powered ultralow-cost paper centrifuge, or paperfuge." With rotational speeds of up to 125,000 revolutions per minute, the device separates blood plasma from red cells in 1.5 minutes, no electricity required. A centrifuge is critical for detecting diseases such as malaria, African sleeping sickness, HIV and tuberculosis. This low-cost version will enable precise diagnosis and treatment in the poor, off-the-grid regions where these diseases are most prevalent. Stanford.
Analytical Services Laboratories are staffed by trained chemists, material scientists, technicians and laboratory management with years of industry knowledge and expertise. Analytical laboratory testing: identify the chemical makeup or characteristics of a particular sample. Analytical Chemistry.
Examinations - Lab Tests (physical health) - Sensors
Laboratory Analyses is a range of analytical parameters to provide the necessary evidence for environmental regulatory compliance and comparative data that can be used to discover ways to minimize environmental impact.
Hollow-core fiber raises prospects for next-generation scientific instruments. Hollow-core optical fibres combine the free-space propagation performance of the most advanced interferometers with the length scales of modern optical fibres by guiding light around bends in an air or vacuum filled core.
Nanodots - Magnetic Constructors - Video
Chem 4 Kids - Molecular Flipbook - Engineering
Toxicology - Pharmaceutical Industry
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures in the absence of oxygen (or any halogen). It involves the simultaneous change of chemical composition and physical phase, and is irreversible.
Bioanalysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics (drugs and their metabolites, and biological molecules in unnatural locations or concentrations) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems.
Janet Iwasa: How 3D Animations help Scientists Visualize what we can't See (video)
Pub Chem provides information on the biological activities of small molecules. Biological properties component database with a fast chemical structure similarity search tool.
Typical Contents found in Chemistry Sets
Equipment might include: vials of dry chemicals, metal wires, such as copper, nickel or zinc, metal filings such as iron, graphite rods, a balance and weights, a measuring cylinder, a thermometer, a magnifying glass, pipettes, beakers, retorts, flasks, test tubes, U-tubes or other reaction vessels, cork stoppers, watch glasses, glass and rubber tubing, test tube holders, retort stands and clamps, an alcohol burner or other heat source, a filter funnel and filter paper, universal indicator paper or litmus paper, safety goggles, an instruction manual.
Eppendorf Tubes are single-use tubes made from polypropylene for preparing, mixing, centrifuging, transporting and storing solid and liquid samples and reagents. The product can be used for training, routine and research laboratories in the areas of life sciences, industry or chemistry.
Chemicals might include: Aluminium ammonium sulfate, Aluminium sulfate, Ammonium chloride, Borax, Calcium chloride, Calcium hydroxide, Calcium oxide, Calcium oxychloride, Calcium sulfate, Cobalt chloride, Cupric chloride, Copper sulfate, Ferric ammonium sulfate, Ferrous sulfate, Gum arabic, Magnesium ribbon, Magnesium chloride Magnesium sulfate, Manganese sulfate, Phenolphthalein, Potassium chloride, Potassium iodide, Potassium permanganate, Potassium sulfate Powdered charcoal, Powdered iron, Sodium bisulfate, Sodium bisulfite, Sodium carbonate, Sodium ferrocyanide, Sodium silicate, Sodium thiosulfate, Strontium chloride, Sulfur, Tannic acid, Tartaric acid, Zinc sulfate.. The experiments described in the instruction manual typically require a number of chemicals not shipped with the chemistry set, because they are common household chemicals: Acetic acid (in vinegar), Ammonium carbonate ("baker's ammonia" or "salts of hartshorn"), Citric acid (in lemons), Ethanol (in denatured alcohol), Sodium bicarbonate, (baking soda), Sodium chloride ("table salt") Other chemicals, including strong acids, bases and oxidizers cannot be safely shipped with the set and others having a limited shelf life have to be purchased separately from a drug store: Hydrochloric acid, Hydrogen peroxide, Silver nitrate,, Sodium hydroxide.
List of Commonly Available Chemicals (wiki)
Amateur Chemistry is the pursuit of chemistry as a private hobby. Amateur chemistry is usually done with whatever chemicals are available at disposal at the privacy of one's home. It should not be confused with clandestine chemistry, which involves the illicit production of controlled drugs. Notable amateur chemists include Oliver Sacks and Sir Edward Elgar.
Chemistry Resources
American Chemical Society - American Chemical Society (youtube channel)
The American Oil Chemists’ Society - American Chemistry
Journals & Publications
Industrial & Engineering Chemistry Research
Chemical Heritage Foundation
Royal Society of Chemistry - Royal Society (wiki)
Related Subject Pages - Biology - Cells - Plants - Animals - Insects - Land - Environment - Science - Vitamins.
Alchemy - Converting Matter
Alchemy is change of one substance into another. Transmutation of metals aimed to purify, mature, and perfect certain objects.
Transmutation is the action of changing or the state of being changed into another form. It is the changing of one element into another by radioactive decay, nuclear bombardment, or similar processes. It is the conversion or transformation of one species into another. Nuclear Transmutation is the conversion of one chemical element or an isotope into another chemical element. Because any element or isotope of one is defined by its number of protons and neutrons in its atoms, i.e. in the atomic nucleus, nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus is changed. States of Matter.
Alchemical Symbol - According to Paracelsus (1493–1541), the three primes or tria prima – of which material substances are immediately composed – are Mercury (Mind) ☿ - Salt (base matter or body) - Sulfur (Soul). Western alchemy makes use of the Hermetic elements. The symbols used for these are: Air, Earth, Fire, Water. Alchemy Symbols.
Seven Planetary Metals - Lead dominated by Saturn ♄ - Tin dominated by Jupiter ♃ -Iron dominated by Mars ♂ - Gold dominated by Sol ☉ - Copper dominated by Venus ♀ - Mercury (quicksilver) dominated by Mercury ☿ - Silver dominated by Moon ☽.
Mundane Elements - Antimony ♁ - Arsenic - Bismuth - Boron - Lithium - Magnesium - Phosphorus - Platinum ☽☉ - Potassium -
Sulfur - Zinc.
Alchemical Compounds - Sal ammoniac (ammonium chloride) - Aqua fortis (nitric acid) - Aqua regia (nitro-hydrochloric acid) - Spirit of wine (concentrated ethanol; also called aqua vitae) - Amalgam (alloys of a metal and mercury) - Cinnabar (mercury sulfide) - Vitriol (sulfates).
Philosopher Stone is a legendary alchemical substance capable of turning base metals such as mercury into gold or silver. It is also called the elixir of life, useful for rejuvenation and for achieving immortality; for many centuries, it was the most sought goal in alchemy. The philosophers' stone was the central symbol of the mystical terminology of alchemy, symbolizing perfection at its finest, enlightenment, and heavenly bliss. Efforts to discover the philosophers' stone were known as the Magnum Opus ("Great Work"). Philosopher King.
Metamaterial is a material engineered to have a property that is not found in nature. They are made from assemblies of multiple elements fashioned from composite materials such as metals or plastics. Bio-Mimicry.
Metallurgy (metal working) - Materials Science (load capacities and strength).
Base Metal is a common and inexpensive metal, as opposed to a precious metal such as gold or silver. A long-time goal of alchemists was the transmutation of a base (low grade) metal into a precious metal. In numismatics, coins often derived their value from the precious metal content; however, base metals have also been used in coins in the past and today.