BK101
Knowledge Base
Batteries - Potential Electrical Energy Storage
Battery Types - Energy Storage - Recycling
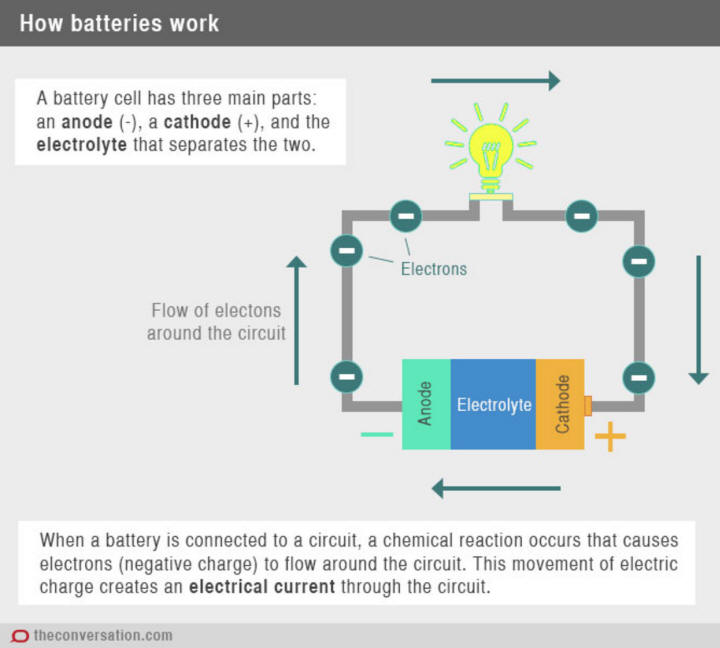 Battery is an electrical energy storage device consisting of one or more
electrochemical cells with external connections
that provide power
to electrical devices such as flashlights,
smartphones, and
electric cars.
When a battery is supplying electric power, its positive terminal is the
cathode and its negative terminal is the
anode. The terminal marked
negative is the source of electrons that when connected to an external
circuit will flow and deliver energy to an external device. When a battery
is connected to an external circuit, electrolytes are able to move as ions
within, allowing the chemical reactions to be completed at the separate
terminals and so deliver energy to the external circuit. It is the
movement of those ions within the battery which allows current to flow out
of the battery to perform work. Historically the term "battery"
specifically referred to a device composed of multiple cells, however the
usage has evolved to additionally include devices composed of a single cell.
Battery is an electrical energy storage device consisting of one or more
electrochemical cells with external connections
that provide power
to electrical devices such as flashlights,
smartphones, and
electric cars.
When a battery is supplying electric power, its positive terminal is the
cathode and its negative terminal is the
anode. The terminal marked
negative is the source of electrons that when connected to an external
circuit will flow and deliver energy to an external device. When a battery
is connected to an external circuit, electrolytes are able to move as ions
within, allowing the chemical reactions to be completed at the separate
terminals and so deliver energy to the external circuit. It is the
movement of those ions within the battery which allows current to flow out
of the battery to perform work. Historically the term "battery"
specifically referred to a device composed of multiple cells, however the
usage has evolved to additionally include devices composed of a single cell.
Negative charge repels, and energy flows outward. Electrons have a negative charge and can be easily moved between atoms. Positive charge attracts, and energy flows inward. Protons have a positive charge and cannot move between atoms. When you use a cloth to rub an insulator such as a balloon or a plastic ruler, electrons are rubbed from one to the other.
Make an AA Battery (youtube) - Electric Potential Difference (voltage).
Baghdad Battery is a set of three artifacts which were found together: a ceramic pot, a tube of copper, and a rod of iron. It was discovered in modern Khujut Rabu, Iraq, close to the metropolis of Ctesiphon, the capital of the Parthian (150 BC – 223 AD) and Sasanian (224–650 AD) empires, and it is considered to date from either of these periods. Its origin and purpose remain unclear. It was hypothesized by some researchers that the object functioned as a galvanic cell, possibly used for electroplating, or some kind of electrotherapy, but there is no electroplated object known from this period. An alternative explanation is that it functioned as a storage vessel for sacred scrolls. History of the Battery (wiki). Egyptian Pyramids (wiki).
Voltaic Pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Alessandro Volta, who published his experiments in 1799. The voltaic pile then enabled a rapid series of discoveries.
Electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air).
Cathode is the electrode from which a conventional current leaves a polarized electrical device. (This definition can be recalled by using the mnemonic CCD for cathode current departs.) A conventional current describes the direction in which positive electronic charges move. Electrons have a negative charge, so the movement of electrons is opposite to the conventional current flow. Consequently, the mnemonic cathode current departs also means that electrons flow into the device's cathode. Hybrid Cathodes (MIT).
Anode is an electrode through which conventional current flows into a polarized electrical device. A common mnemonic is ACID for "anode current into device". The direction of (positive) electric current is opposite to the direction of electron flow: (negatively charged) electrons flow out the anode to the outside circuit.
Electrolyte is a substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water. The dissolved electrolyte separates into cations and anions, which disperse uniformly through the solvent. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. This includes most soluble salts, acids, and bases. Some gases, such as hydrogen chloride, under conditions of high temperature or low pressure can also function as electrolytes. Electrolyte solutions can also result from the dissolution of some biological (e.g., DNA, polypeptides) and synthetic polymers (e.g., polystyrene sulfonate), termed "polyelectrolytes", which contain charged functional groups. A substance that dissociates into ions in solution acquires the capacity to conduct electricity. Sodium, potassium, chloride, calcium, magnesium, and phosphate are examples of electrolytes, informally known as "lytes". Capacitors - Body Electrolytes.
Giant Charge Reversal observed for the first time. Charged surfaces submerged in an electrolyte solution can sometimes become oppositely charged.
Battery's Hidden Layer Revealed. Microscopically thin layer that forms between the liquid electrolyte and solid electrode in lithium-ion batteries.
Seeing 'under the hood' in batteries. A high-sensitivity X-ray technique is attracting a growing group of scientists because it provides a deep, precise dive into battery chemistry and how the individual ingredients of battery materials behave beneath the surface.
Paper-thin gallium oxide transistor handles more than 8,000 volts. The transistor could lead to smaller and more efficient electronic systems that control and convert electric power -- a field of study known as power electronics -- in electric cars, locomotives and airplanes. In turn, this could help improve how far these vehicles can travel.
Million Mile Battery - If you drive your electric car 25 miles a day or drive 200 miles a week, that would be around 800 miles a month or 10,000 miles a year. So your car battery will last 100 years.
Electro Chemistry
Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as an oxidation-reduction ("redox") reaction. It is the branch of physical chemistry that studies the relationship between electricity, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electricity considered an outcome of a particular chemical change or vice versa. These reactions involve electric charges moving between electrodes and an electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change. When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte. Electro-Chemistry.
Electrochemistry deals with the interaction between electrical energy and chemical change. When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction.
Electrochemical Production of Glycolic Acid from Oxalic Acid Using a Polymer Electrolyte Alcohol Electrosynthesis Cell Containing a Porous TiO2 Catalyst.
Bio-Electro-Chemistry is a branch of electrochemistry and biophysical chemistry concerned with electrophysiological topics like cell electron-proton transport, cell membrane potentials and electrode reactions of redox enzymes.
Electric Nature - Bio- Battery - Electrolysis
is a technique that uses a direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential, which is the minimum voltage (difference in electrode potential) between anode and cathode of an electrolytic cell that is needed for electrolysis to occur.
Hydrolysis is a term used for both an electro-chemical process and a biological one. The hydrolysis of water is the separation of water molecules into hydrogen and oxygen atoms (water splitting) using electricity (electrolysis). Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g. sucrose being broken down into glucose and fructose), this is termed saccharification. Generally, hydrolysis or saccharification is a step in the degradation of a substance. Hydrolysis can be the reverse of a condensation reaction in which two molecules join together into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water and any other solvents. Some hydration reactions are hydrolysis.
Electrolytic Cell is an electrochemical cell that drives a non-spontaneous redox reaction through the application of electrical energy. They are often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means to break up.
Electrophoretic Deposition is a term for a broad range of industrial processes which includes electrocoating, cathodic electrodeposition, anodic electrodeposition, and electrophoretic coating, or electrophoretic painting. A characteristic feature of this process is that colloidal particles suspended in a liquid medium migrate under the influence of an electric field (electrophoresis) and are deposited onto an electrode. All colloidal particles that can be used to form stable suspensions and that can carry a charge can be used in electrophoretic deposition. This includes materials such as polymers, pigments, dyes, ceramics and metals.
Electroplating is a process that uses electric current to reduce dissolved metal cations so that they form a thin coherent metal coating on an electrode. The term is also used for electrical oxidation of anions on to a solid substrate, as in the formation silver chloride on silver wire to make silver/silver-chloride electrodes. Electroplating is primarily used to change the surface properties of an object (such as abrasion and wear resistance, corrosion protection, lubricity, aesthetic qualities), but may also be used to build up thickness on undersized parts or to form objects by electroforming.
Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst.
Chemical Energy is the potential of a chemical substance to undergo a transformation through a chemical reaction to transform other chemical substances. Examples include batteries, food, gasoline, and more. Breaking or making of chemical bonds involves energy, which may be either absorbed or evolved from a chemical system. Energy that can be released (or absorbed) because of a reaction between a set of chemical substances is equal to the difference between the energy content of the products and the reactants, if the initial and final temperatures are the same. This change in energy can be estimated from the bond energies of the various chemical bonds in the reactants and products.
Bio-Batteries - Fuel Cells - Photosynthesis - Chemical Synthesis.
Building a better battery with machine learning. Argonne researchers first created a highly accurate database of roughly 133,000 small organic molecules that could form the basis of battery electrolytes. Because using G4MP2 to resolve each of the 166 billion molecules would have required an impossible amount of computing time and power, the research team used a machine learning algorithm to relate the precisely known structures from the smaller data set to much more coarsely modeled structures from the larger data set. The machine learning algorithm gives us a way to look at the relationship between the atoms in a large molecule and their neighbors, to see how they bond and interact, and look for similarities between those molecules and others we know quite well.
Capacitors
Capacitor is a passive two-terminal electrical component that stores electrical energy in an electric field. The effect of a capacitor is known as capacitance. While capacitance exists between any two electrical conductors of a circuit in sufficiently close proximity, a capacitor is specifically designed to provide and enhance this effect for a variety of practical applications by consideration of size, shape, and positioning of closely spaced conductors, and the intervening dielectric material. A capacitor was therefore historically first known as an electric condenser. Static Electricity.
Energy Density is the amount of energy stored in a given system or region of space per unit volume or mass, though the latter is more accurately termed specific energy. Often only the useful or extractable energy is measured, which is to say that chemically inaccessible energy such as rest mass energy is ignored. Food Energy.
Power Density is the amount of power (time rate of energy transfer) per unit volume. In energy transformers including batteries, fuel cells, motors, etc., and also power supply units or similar, power density refers to a volume. It is then also called volume power density, which is expressed as W/m3. Volume power density is sometimes an important consideration where space is constrained. In reciprocating internal combustion engines, power density—power per swept volume or brake horsepower per cubic centimeter —is an important metric. This is based on the internal capacity of the engine, not its external size.
Energy Transfer is the portion of the energy which is transferred by conservative forces over a distance and is measured as the work the source system does on the receiving system. The portion of the energy which does not do work during the transfer is called heat. Energy can be transferred between systems in a variety of ways. Examples include the transmission of electromagnetic energy via photons, physical collisions which transfer kinetic energy, and the conductive transfer of thermal energy.
Electric Double-Layer Capacitor are electrochemical capacitors which energy storage predominant is achieved by Double-layer capacitance. In the past, all electrochemical capacitors were called "double-layer capacitors". However, since some years it is known that double-layer capacitors together with pseudocapacitors are part of a new family of electrochemical capacitors called supercapacitors, also known as ultracapacitors. Supercapacitors do not have a conventional solid dielectric. The capacitance value of a supercapacitor is determined by two storage principles: Double-layer capacitance – electrostatic storage of the electrical energy achieved by separation of charge in a Helmholtz double layer at the interface between the surface of a conductor electrode and an electrolytic solution electrolyte. The separation of charge distance in a double-layer is on the order of a few Ångströms (0.3–0.8 nm) and is static in origin. Pseudocapacitance – Electrochemical storage of the electrical energy, achieved by redox reactions electrosorption or intercalation on the surface of the electrode by specifically adsorbed ions that results in a reversible faradaic charge-transfer on the electrode.
Supercapacitor is a high-capacity electrochemical capacitor with capacitance values much higher than other capacitors (but lower voltage limits) that bridge the gap between electrolytic capacitors and rechargeable batteries. They typically store 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerate many more charge and discharge cycles than rechargeable batteries.
New Materials for High-Voltage Supercapacitors. The new material has an energy density 2.7 times higher than conventional materials. MIT uses neutrons in drive to improve supercapacitors.
Micro-supercapacitors possess remarkable features of high electrochemical performance and relatively small volume are promising candidates for energy storage in micro-devices.
Surface-Active Ionic Liquid Cholinium Dodecylbenzenesulfonate: Self-Assembling Behavior and Interaction with Cellulase.
Candy cane Super-Capacitor could enable fast charging of mobile phones
Kilowatt Labs Supercapacitor delivers deep cycle discharge, long duration discharge as well as fast charge / short discharge, along with all the inherent advantages supercapacitors have over conventional chemical batteries.
Hemp based Supercapacitor (youtube)
Skeleton Ultra Capacitors with Graphene.
Double Layer in surface science is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on the scale of micrometres to nanometres. However, DLs are important to other phenomena, such as the electrochemical behaviour of electrodes. DLs play a fundamental role in many everyday substances. For instance, homogenized milk exists only because fat droplets are covered with a DL that prevents their coagulation into butter. DLs exist in practically all heterogeneous fluid-based systems, such as blood, paint, ink and ceramic and cement slurry. The DL is closely related to electrokinetic phenomena and electroacoustic phenomena. Helmholtz.
Surface Science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.
Parallel-Plate Model is the simplest model capacitor that consists of two thin parallel conductive plates each with an area of A separated by a uniform gap of thickness d filled with a dielectric with permittivity ε. It is assumed the gap d is much smaller than the dimensions of the plates. This model applies well to many practical capacitors which are constructed of metal sheets separated by a thin layer of insulating dielectric, since manufacturers try to keep the dielectric very uniform in thickness to avoid thin spots which can cause failure of the capacitor.
Permittivity is the measure of capacitance that is encountered when forming an electric field in a particular medium. More specifically, permittivity describes the amount of charge needed to generate one unit of electric flux in a particular medium. Accordingly, a charge will yield more electric flux in a medium with low permittivity than in a medium with high permittivity. Permittivity is the measure of a material's ability to store an electric field in the polarization of the medium. The SI unit for permittivity is farad per meter (F/m or F·m−1). Permittivity of Space (wiki).
Flexible Super-Capacitors that can store more energy and be recharged more than 30,000 times without degrading.
3-D Surface-Microporous Graphene material's surface is pockmarked with micropores and folds into larger mesopores, which both increase the surface area available for adsorption of electrolyte ions. It would be an excellent electrode material for energy storage devices. The interconnected mesopores are channels that can act as an electrolyte reservoir and the surface-micropores adsorb electrolyte ions without needing to pull the ions deep inside the micropore. To synthesize the material from carbon dioxide, Hu's team added carbon dioxide to sodium, followed by increasing temperature to 520 degrees Celsius. The reaction can release heat instead of require energy input. During the process, carbon dioxide not only forms 3-D graphene sheets, but also digs the micropores. The tiny dents are only 0.54 nanometers deep in the surface layers of graphene.
Conductive electrodes are key to fast-charging batteries fully charging your cell phone in just a few seconds.
MXenes are a class of two-dimensional inorganic compounds. These materials consist of few atoms thick layers of transition metal carbides, nitrides, or carbonitrides.
Ultra-Capacitor Buses
Advanced Capacitor Circuits using series and parallel techniques (youtube)
Graphene (nano technology)
Fast-Charging Super-Capacitor Technology. The ATI's super-capacitor technology is based on a material called Polyaniline (PANI), which stores energy through a mechanism known as "pseudocapacitance." This cheap polymer material is conductive and can be used as the electrode in a super-capacitor device. The electrode stores charge by trapping ions within the electrode. It does this by exchanging electrons with the ion, which "dopes" the material.
EEstor Corp - Thermal Energy Storage.
Electrostatics is a branch of physics that deals with the phenomena and properties of stationary or slow-moving electric charges. Static.
Lightweight Green Supercapacitors could charge devices in a jiffy. Researchers have described their novel plant-based energy storage device that could charge even electric cars within a few minutes in the near future. Furthermore, they said their devices are flexible, lightweight and cost-effective.
Energy Storage
Energy Storage is the capture of energy produced at one time for use at a later time.
Flow Battery - Battery Types - Giga Factory - Solar Heat Storage
Grid Energy Storage is a collection of methods used to store electrical energy on a large scale within an electrical power grid.
Rechargeable Batteries provide inexpensive power for industrial-scale storage systems. Battery based on electrodes made of sodium and nickel chloride and using a new type of metal mesh membrane.
Spontaneous formation of nanoscale hollow structures could boost battery storage. An unexpected property of nanometer-scale antimony crystals -- the spontaneous formation of hollow structures -- could help give the next generation of lithium ion batteries higher energy density without reducing battery lifetime. The reversibly hollowing structures could allow lithium ion batteries to hold more energy and therefore provide more power between charges.
Flywheel Energy Storage
New Device could Increase Battery Life of electronics by a Hundred-Fold or 100 times more then before.
The World's Biggest Lithium Ion Battery has started delivering power, providing electricity for as many as 30,000 homes in South Australia. The battery was built in 100 days.
Alacaes Energy Storage uses excess renewable energy to store compress air using adiabatic compressors, then when needed, the direction of the flow is reversed to convert the compressed air back to electricity using turbines. One of the lowest CAPEX per kWh of any storage technology. Thermal Energy Storage System.
Gravitricity is electrical power that is absorbed or generated by raising or lowering a big weight.
Ares uses the power of gravity for grid-scale energy storage.
Pumped-Storage Hydroelectricity is a type of hydroelectric energy storage used by electric power systems for load balancing. The method stores energy in the form of gravitational potential energy of water, pumped from a lower elevation reservoir to a higher elevation. Low-cost surplus off-peak electric power is typically used to run the pumps. During periods of high electrical demand, the stored water is released through turbines to produce electric power. Although the losses of the pumping process makes the plant a net consumer of energy overall, the system increases revenue by selling more electricity during periods of peak demand, when electricity prices are highest. Dams (hydro energy).
Amber Kinetics flywheel energy storage system for utility-scale applications. Perpetual Motion.
Energy Storage Terminology
List of Energy Storage Projects (wiki)
Energy Storage Research
Energy Storage News
Smart Grid Battery Storage - Smart Grid
Ice Bear Distributed Mature Energy Storage Technology
Electricity Storage Technology
Faster, more efficient energy storage could stem from holistic study of layered materials. A team has developed a novel, integrated approach to track energy-transporting ions within an ultra-thin material, which could unlock its energy storage potential leading toward faster charging, longer lasting devices.
Curtailment is the reduction of output of a renewable resource below what it could have otherwise produced. It is calculated by subtracting the energy that was actually produced from the amount of electricity forecasted to be generated.
Portable Backup Battery Power
Solar Powered Battery Backup Electricity (portable) - Portable Solar PowerSolar Energy Batteries
Power Vault is a home electricity storage product which helps all households use energy more efficiently and helps you reduce your energy bills by storing free solar energy or cheap energy from the grid. The online portal allows you to monitor your energy savings and select your smart tariff charging schedule.
Tesla Motors Powerwall - Smart Grid Energy Storage
Salt Water Battery - Absorbent Glass Mat (wiki)
Deepcycle Battery - Deep Cycle GEL - US Battery
12v 155ah Deep Cycle Rechargeable (amazon)
Deep Cycle Battery is a lead-acid battery designed to be regularly deeply discharged using most of its capacity. In contrast, starter batteries (e.g. most automotive batteries) are designed to deliver short, high-current bursts for cranking the engine, thus frequently discharging only a small part of their capacity. While a deep-cycle battery can be used as a starting battery, the lower "cranking current" implies that an oversized battery may be required. A deep-cycle battery is designed to discharge between 45% and 75% of its capacity, depending on the manufacturer and the construction of the battery. Although these batteries can be cycled down to 20% charge, the best lifespan vs cost method is to keep the average cycle at about 45% discharge. There is an indirect correlation between the depth of discharge of the battery, and the number of charge and discharge cycles it can perform.
Charging Batteries - Recharging Batteries
Rechargeable Battery is a type of electrical battery which can be charged, discharged into a load, and recharged many times, while a non-rechargeable or primary battery is supplied fully charged, and discarded once discharged.
Depth of Discharge (DOD) is an alternate method to indicate a battery's state of charge (SOC). The DOD is the complement of SOC: as the one increases, the other decreases. While the SOC units are percent points (0% = empty; 100% = full), DOD can use Ah units (e.g.: 0 = full, 50 Ah = empty) or percent points (100% = empty; 0% = full). As a battery may actually have higher capacity than its nominal rating, it is possible for the DOD value to exceed the full value (e.g.: 55 Ah or 110%), something that is not possible when using state of charge. Not letting your phone get below 50 percent can help extend its life? And not charging to 100 percent too because being charged at 100 percent produces a small amount of heat, and lithium-ion batteries hate heat.
Charging Station supplies electric energy for the recharging of electric vehicles, such as plug-in electric vehicles, including electric cars, neighborhood electric vehicles and plug-in hybrids.
Charging Locations - Charge Point - Battery Switch Station
Sila Nanotechnologies Batteries through higher volumetric energy density Sila materials enable more energy in each cell means fewer cells for the same battery pack capacity and vehicle range, and therefore much lower cost overall.
Instantly Rechargeable Battery could change the future of Electric and Hybrid Automobiles.
Self-Assembling 3D Battery would Charge in Seconds.
Charging electric cars up to 90% in 6 minutes. New Li-ion battery electrode material that can achieve high-energy density and high power capability per volume without reducing particle size.
Battery Research could Triple Range of Electric Vehicles. The breakthrough involves the use of negative electrodes made of lithium metal, a material with the potential to dramatically increase battery storage capacity. This will mean cheap, safe, long-lasting batteries that give people much more range in their electric vehicles.
EMBATT Bipolar Electrode Ceramic Technologies. Individual battery cells are not strung separately side-by-side in small sections; instead, they are stacked directly one above the other across a large area. The entire structure for the housing and the contacting is therefore eliminated. As a result, more batteries fit into the car. Through the direct connection of the cells in the stack, the current flows over the entire surface of the battery. The electrical resistance is thereby considerably reduced.
New Electric Car Batteries (youtube)
Tesla Motors Supercharger - Wireless Charging - Human Energy Charging
Internal Resistance. When the power source delivers current, the measured voltage output is lower than the no-load voltage; the difference is the voltage drop (the product of current and resistance) caused by the internal resistance. The concept of internal resistance applies to all kinds of electrical sources and is useful for analyzing many types of electrical circuits.
Battery Management System is any electronic system that manages a rechargeable battery (cell or battery pack), such as by protecting the battery from operating outside its Safe Operating Area, monitoring its state, calculating secondary data, reporting that data, controlling its environment, authenticating it and / or balancing it. A battery pack built together with a battery management system with an external communication data bus is a smart battery pack. A smart battery pack must be charged by a smart battery charger.
Capacitors
New Breakthrough In Battery Charging Technology. UNIST researchers introduce new battery charging technology that uses light to charge batteries. UNIST has developed a single-unit, photo-rechargeable portable power source based on high-efficiency silicon solar cells and lithium-ion batteries (LIBs). This newly-developed power source is designed to work under sunlight and indoor lighting, allowing users to power their portable electronics anywhere with access to light. In addition, the new device could power electric devices even in the absence of light.
Battery Types
Voltaic Pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Alessandro Volta, who published his experiments in 1800.
Super-Capacitors (energy storage) - Fuel Cells - Rechargeable - Battery Types List (wiki)
Atomic Battery are used to describe a device which uses energy from the decay of a radioactive isotope to generate electricity. Like nuclear reactors, they generate electricity from atomic energy, but differ in that they do not use a chain reaction. Compared to other batteries they are very costly, but have an extremely long life and high energy density, and so they are mainly used as power sources for equipment that must operate unattended for long periods of time, such as spacecraft, pacemakers, underwater systems and automated scientific stations in remote parts of the world.
Betavoltaic Device is also known as betavoltaic cells, are generators of electric current, in effect a form of battery, which use energy from a radioactive source emitting beta particles (electrons). A common source used is the hydrogen isotope, tritium. Unlike most nuclear power sources, which use nuclear radiation to generate heat, which then is used to generate electricity (thermoelectric and thermionic sources), betavoltaics use a non-thermal conversion process; converting the electron-hole pairs produced by the ionization trail of beta particles traversing a semiconductor. Betavoltaic power sources (and the related technology of alphavoltaic power sources) are particularly well-suited to low-power electrical applications where long life of the energy source is needed, such as implantable medical devices or military and space applications.
Diamond Battery is proposed to run on the radioactivity of waste graphite blocks (previously used as neutron moderator material in nuclear reactors) and would last for thousands of years. The battery, developed by the University of Bristol, is a betavoltaic cell using carbon-14 in the form of diamond-like carbon (DLC) as the beta radiation source, and additional normal-carbon DLC to make the necessary semiconductor junction and encapsulate the carbon-14.
Oxford Electric Bell is an experimental electric bell that was set up in 1840 and which has run nearly continuously ever since.
VRLA Battery (valve-regulated lead-acid battery), more commonly known as a sealed lead-acid (SLA), gel cell, or maintenance free battery, is a type of lead-acid rechargeable battery.
Alkaline Battery are a type of primary battery dependent upon the reaction between zinc and manganese(IV) oxide (Zn/MnO2). A rechargeable alkaline battery allows reuse of specially designed cells.
Batteriser: Extend Battery Life by 8X
Lead Acid Battery despite having a very low energy-to-weight ratio and a low energy-to-volume ratio, its ability to supply high surge currents means that the cells have a relatively large power-to-weight ratio. These features, along with their low cost, makes it attractive for use in motor vehicles to provide the high current required by automobile starter motors.
Nickel Cadmium Battery is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes. The abbreviation NiCd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd): the abbreviation NiCad is a registered trademark of SAFT Corporation, although this brand name is commonly used to describe all Ni–Cd batteries.
Prismatic Cells are encased in aluminum or steel for stability. Jelly-rolled or stacked, the cell is space-efficient but can be costlier to manufacture than the cylindrical cell. Modern prismatic cells are used in the electric powertrain and energy storage systems.
Cylindrical Batteries are round batteries with height longer than their diameter.
Solid-State Batteries
Aluminum Air Battery produces electricity from the reaction of oxygen in the air with aluminium. They have one of the highest energy densities of all batteries, but they are not widely used because of problems with high anode cost and byproduct removal when using traditional electrolytes. This has restricted their use to mainly military applications. However, an electric vehicle with aluminium batteries has the potential for up to eight times the range of a lithium-ion battery with a significantly lower total weight. Aluminium–air batteries are primary cells, i.e., non-rechargeable. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water-based electrolyte to form hydrated aluminium oxide, the battery will no longer produce electricity. However, it is possible to mechanically recharge the battery with new aluminium anodes made from recycling the hydrated aluminium oxide. Such recycling would be essential if aluminium–air batteries are to be widely adopted.
Power Density is the amount of power (time rate of energy transfer) per unit volume. Capacitors.
Energy Density is the amount of energy stored in a given system or region of space per unit volume.
Lithium Polymer Battery is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. High conductivity semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, like mobile devices and radio-controlled aircraft.
Lithium-ion Batteries is a type of rechargeable battery in which lithium ions move from the negative electrode to the positive electrode during discharge and back when charging. Li-ion batteries use an intercalated lithium compound as one electrode material, compared to the metallic lithium used in a non-rechargeable lithium battery. The electrolyte, which allows for ionic movement, and the two electrodes are the constituent components of a lithium-ion battery cell. Lithium iron phosphate (LiFePO4), lithium ion manganese oxide battery (LiMn2O4, Li2MnO3, or LMO) and lithium nickel manganese cobalt oxide (LiNiMnCoO2 or NMC) offer lower energy density, but longer lives and inherent safety. Such batteries are widely used for electric tools, medical equipment and other roles. NMC in particular is a leading contender for automotive applications. Lithium nickel cobalt aluminum oxide (LiNiCoAlO2 or NCA) and lithium titanate (Li4Ti5O12 or LTO) are specialty designs aimed at particular niche roles. The newer lithium–sulfur batteries promise the highest performance-to-weight ratio. Dendrites.
Lithium-ion Battery Electrode Protection
Thermal Runaway (feedback effects)
Lithium-Oxygen Battery which has very high energy density, is more than 90% efficient, and, to date, can be recharged more than 2000 times
Photoelectrode Lithium–Oxygen Battery.
Predicting the slow death of lithium-ion batteries. Stanford technology predicts the slow death of lithium-ion batteries.
Lithium-Carbon Dioxide Batteries consist of two electrodes—an anode made of lithium and a cathode made of carbon—and an electrolyte that shuttles charged particles between the electrodes as the battery is charged and discharged. lithium carbonate and carbon build up in the catalyst and slowly destroys the battery. But this problem is being fixed, which would make the battery last 7 times longer.
New Coating could have big implications for Lithium Batteries. Coating provides extra layer of protection for battery cathodes. Nickel-manganese-cobalt cathode material and encapsulated them with a sulfur-containing polymer called PEDOT. This polymer provides the cathode a layer of protection from the battery's electrolyte as the battery charges and discharges.
Liquid Microscopy technique reveals new problem with Lithium-Oxygen Batteries. Lithium Peroxide develops in the liquid electrolyte of lithium-oxygen batteries, and is a contributor to the slow down and ultimate death of these batteries.
Kair Battery is a breathing solar battery that recharges itself with air and light.
New Lithium-Rich Battery could last much longer. Battery leverages both iron and oxygen to drive more lithium ions.
Lithium-Ion Batteries for extreme environments.
Single-crystal technology holds promise for next-generation lithium-ion batteries. Scientists have improved a promising battery technology, creating a single-crystal, nickel-rich cathode that is hardier and more efficient than before. It's one step toward improved lithium-ion batteries that are common in electric vehicles today. Increasing nickel content in the cathode is on the drawing board of lithium-ion battery makers largely because of its relatively low cost, wide availability and low toxicity compared to other key battery materials, such as cobalt.
New class of cobalt-free cathodes could enhance energy density of next-gen lithium-ion batteries. Researchers have developed a new family of cathodes with the potential to replace the costly cobalt-based cathodes typically found in today's lithium-ion batteries that power electric vehicles and consumer electronics. The new class called NFA, which stands for nickel-, iron- and aluminum-based cathode, is a derivative of lithium nickelate and can be used to make the positive electrode of a lithium-ion battery. These novel cathodes are designed to be fast charging, energy dense, cost effective, and longer lasting.
Organic Lithium Batteries Operated at −70°C. Researchers in China have developed a battery with organic compound electrodes that can function at -70 degrees Celsius.
Next-gen Lithium-Metal Batteries for electric vehicles, smart grids. Using supercomputers, researchers have simulated the behavior of graphene oxide nanosheets that can limit the formation of dendrites.
Prieto Battery 3D Lithium-Ion Battery Technology that will deliver transformational performance. Very high power density, long cycle life, Safe, Greater energy density.
Lithium Sulfur Battery is a type of rechargeable battery, notable for its high specific energy. The low atomic weight of lithium and moderate weight of sulfur means that Li–S batteries are relatively light (about the density of water).
Fast Charging Lithium-ion Battery
Lithium Iron Phosphate Battery. The lithium iron phosphate battery (LiFePO4 battery) or LFP battery (lithium ferrophosphate), is a type of rechargeable battery, specifically a lithium-ion battery, using LiFePO4 as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. The specific capacity of LiFePO4 is higher than that of the related lithium cobalt oxide (LiCoO2) chemistry, but its energy density is less due to its lower operating voltage. The main drawback of LiFePO4 is its low electrical conductivity. Therefore, all the LiFePO4 cathodes under consideration are actually LiFePO4/C. Because of low cost, low toxicity, well-defined performance, long-term stability, etc. LiFePO4 is finding a number of roles in vehicle use, utility scale stationary applications, and backup power. $45 LiFePo4 Cells for your DIY Powerwall (youtube) - Build a DIY Lithium LiFePo4 Headway 12v Battery replacement (youtube).
Asphalt may help high-capacity Lithium Metal Batteries charge 10 to 20 times faster than commercial lithium-ion batteries.
Lithium Air Battery is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
First Fully Rechargeable Carbon Dioxide Battery with Carbon Neutrality. Researchers are the first to show that lithium-carbon dioxide batteries can be designed to operate in a fully rechargeable manner, and they have successfully tested a lithium-carbon dioxide battery prototype running up to 500 consecutive cycles of charge/recharge processes.
Extending the life of low-cost, compact, lightweight batteries. Metal-air batteries are one of the lightest and most compact types of batteries available, but they can have a major limitation: When not in use, they degrade quickly, as corrosion eats away at their metal electrodes. Now, MIT researchers have found a way to substantially reduce that corrosion, making it possible for such batteries to have much longer shelf lives.While typical rechargeable lithium-ion batteries only lose about 5 percent of their charge after a month of storage, they are too costly, bulky, or heavy for many applications. Primary (nonrechargeable) aluminum-air batteries are much less expensive and more compact and lightweight, but they can lose 80 percent of their charge a month. The MIT design overcomes the problem of corrosion in aluminum-air batteries by introducing an oil barrier between the aluminum electrode and the electrolyte -- the fluid between the two battery electrodes that eats away at the aluminum when the battery is on standby. The oil is rapidly pumped away and replaced with electrolyte as soon as the battery is used. As a result, the energy loss is cut to just 0.02 percent a month -- more than a thousandfold improvement. A key to the new system is a thin membrane placed between the battery electrodes. When the battery is in use, both sides of the membrane are filled with a liquid electrolyte, but when the battery is put on standby, oil is pumped into the side closest to the aluminum electrode, which protects the aluminum surface from the electrolyte on the other side of the membrane. The new battery system also takes advantage of a property of aluminum called "underwater oleophobicity" -- that is, when aluminum is immersed in water, it repels oil from its surface. As a result, when the battery is reactivated and electrolyte is pumped back in, the electrolyte easily displaces the oil from the aluminum surface, which restores the power capabilities of the battery. Ironically, the MIT method of corrosion suppression exploits the same property of aluminum that promotes corrosion in conventional systems.
Metal Air Electrochemical Cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte. During discharging of a metal–air electrochemical cell, an oxygen reduction reaction occurs in the ambient air cathode while the metal anode is oxidized. The specific capacity and energy density of metal–air electrochemical cells is higher than that of lithium-ion batteries, making them a prime candidate for use in electric vehicles. However, complications associated with the metal anodes, catalysts, and electrolytes have hindered development and implementation of metal–air batteries.
Reversible Nitrogen Fixation Based on a Rechargeable Lithium-Nitrogen Battery for Energy Storage. A rechargeable Li-N2 battery is proposed for a reversible N2 fixation process. The Li-N2 battery provides technological progress in N2 fixation. The Li-N2 battery shows high faradic efficiency for N2 fixation. The catalyst can improve faradic efficiency and decrease energy consumption.
Lithium Cobalt Oxide is a chemical compound commonly used in the positive electrodes of lithium-ion batteries.
Safe Rechargeable Battery using Glass Electrolytes, substitution of low-cost sodium for lithium sodium is extracted from seawater that is widely available.
Glass Battery is a type of solid state battery. It uses a glass electrolyte and lithium or sodium metal electrodes.
Anode-Free Zinc Battery that could someday store renewable energy. Researchers have made a prototype of an anode-free, zinc-based battery that uses low-cost, naturally abundant materials. Researchers used a manganese dioxide cathode that they pre-intercalated with zinc ions, an aqueous zinc trifluoromethanesulfonate electrolyte solution and a copper foil current collector. During charging, zinc metal gets plated onto the copper foil, and during discharging the metal is stripped off, releasing electrons that power the battery. To prevent dendrites from forming, the researchers coated the copper current collector with a layer of carbon nanodiscs. This layer promoted uniform zinc plating, thereby preventing dendrites, and increased the efficiency of zinc plating and stripping. The battery showed high efficiency, energy density and stability, retaining 62.8% of its storage capacity after 80 charging and discharging cycles. The anode-free battery design opens new directions for using aqueous zinc-based batteries in energy storage systems.
Solid State Batteries
Solid-State Battery is a battery that has both solid electrodes and solid electrolytes. As a group, these materials are very good conductors of ions, which is necessary for good electrolyte and electrode performance, and are essentially insulating toward electrons, which is desirable in electrolytes but undesirable in electrodes. The high ionic conductivity minimizes the internal resistance of the battery, thus permitting high power densities, while the high electronic resistance minimizes its self-discharge rate, thus enhancing its charge retention. Solid State Energy - SiC Nano (youtube) - Sakti 3.
All Solid State Lithium Batteries with Solid Electrolytes
Newly-Developed Solid-Electrolyte Interphase (SEI) aims to improve Lithium Metal Battery Life and Safety.
Semisolid Lithium-Ion - Lithium Ceramic Battery
Solid-State Physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. It also has direct applications, for example in the technology of transistors and semiconductors.
Pouch Cell makes the most efficient use of space and achieves a 90 to 95 percent packaging efficiency, the highest among battery packs. Eliminating the metal enclosure reduces weight but the cell needs some alternative support in the battery compartment. Rather than using a metallic cylinder and glass-to-metal electrical feed-through for insulation, conductive foil tabs welded to the electrode and sealed to the pouch carry the positive and negative terminals to the outside. Figure 1 illustrates such a pouch cell. Pouch Cell offers a simple, flexible and lightweight solution to battery design. Pouch packs are normally Li-polymer. The energy density can be lower and be less durable than Li-ion in the cylindrical package.
Battery that can be Bent, Stretched and Twisted. For applications in bendable electronic devices, this is precisely the kind of battery they need. Smart clothing items make use of wearable micro-devices or sensors to monitor bodily functions. The two current collectors for the anode and the cathode consist of bendable polymer composite that contains electrically conductive carbon and that also serves as the outer shell. On the interior surface of the composite, the researchers applied a thin layer of micronsized silver flakes. Due to the way the flakes overlap like roof tiles, they don't lose contact with one another when the elastomer is stretched. This guarantees the conductivity of the current collector even if it is subjected to extensive stretching. And in the event that the silver flakes do in fact lose contact with each other, the electrical current can still flow through the carbon-containing composite, albeit more weakly. With the help of a mask, the researchers then sprayed anode and cathode powder onto a precisely defined area of the silver layer. The cathode is composed of lithium manganese oxide and the anode is a vanadium oxide. Water-based electrolyte gel is environmentally more friendly than the commercial electrolytes. In the final step, the scientists stacked the two current collectors with the applied electrodes on top of each other, separated by a barrier layer similar to a picture frame, while the gap in the frame was filled with the electrolyte gel.
High Performance Solid-State Sodium-Ion Battery. Organic cathode offers more reliable contact with electrolyte, a key to stability. Salt Battery.
John B. Goodenough is a German-born American professor and solid-state physicist. He is currently a professor of mechanical engineering and materials science at The University of Texas at Austin. He is widely credited for the identification and development of the Li-ion rechargeable battery as well as for developing the Goodenough–Kanamori rules for determining the sign of the magnetic superexchange in materials. In 2014, he received the Charles Stark Draper Prize for his contributions to the lithium-ion battery.
Paper Battery is an electric battery engineered to use a spacer formed largely of cellulose (the major constituent of paper). It incorporates [nanoscopic scale nanoscale] structures to act as high surface-area electrodes to improve conductivity. In addition to being unusually thin, paper batteries are flexible and environmentally-friendly, allowing integration into a wide range of products. Their functioning is similar to conventional chemical batteries with the important difference that they are non-corrosive and do not require extensive housing.
Paper Battery Powered by Bacteria. A paper battery was made by printing thin layers of metals and other materials onto a paper surface. Then, they placed freeze-dried "exoelectrogens" on the paper. Exoelectrogens are a special type of bacteria that can transfer electrons outside of their cells. The electrons, which are generated when the bacteria make energy for themselves, pass through the cell membrane. They can then make contact with external electrodes and power the battery. To activate the battery, the researchers added water or saliva. Within a couple of minutes, the liquid revived the bacteria, which produced enough electrons to power a light-emitting diode and a calculator. The researchers also investigated how oxygen affects the performance of their device. Oxygen, which passes easily through paper, could soak up electrons produced by the bacteria before they reach the electrode. The team found that although oxygen slightly decreased power generation, the effect was minimal. This is because the bacterial cells were tightly attached to the paper fibers, which rapidly whisked the electrons away to the anode before oxygen could intervene.
Superexchange is the strong (usually) antiferromagnetic coupling between two next-to-nearest neighbour cations through a non-magnetic anion. In this way, it differs from direct exchange in which there is coupling between nearest neighbor cations not involving an intermediary anion. Superexchange is a result of the electrons having come from the same donor atom and being coupled with the receiving ions' spins. If the two next-to-nearest neighbor positive ions are connected at 90 degrees to the bridging non-magnetic anion, then the interaction can be a ferromagnetic interaction.Superexchange was proposed by Hendrik Kramers in 1934 when he noticed that in crystals like MnO, there are Mn atoms that interact with one another despite having nonmagnetic oxygen atoms between them (Fig. 1). Phillip Anderson later refined Kramers' model in 1950.
Antiferromagnetism is the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. Generally, antiferromagnetic order may exist at sufficiently low temperatures, but vanishes at and above the Néel temperature – named after Louis Néel, who had first identified this type of magnetic ordering. Above the Néel temperature, the material is typically paramagnetic.
All-Solid-State Polymer Electrolyte with Plastic Crystal Materials for Rechargeable Lithium-ion Battery
Sodium Sulfur Battery s a type of molten-salt battery constructed from liquid Sodium (Na) and sulfur (S). This type of battery has a high energy density, high efficiency of charge/discharge (89–92%) and long cycle life, and is fabricated from inexpensive materials. The operating temperatures of 300 to 350 °C and the highly corrosive nature of the sodium polysulfides, primarily make them suitable for stationary energy storage applications. The cell becomes more economical with increasing size. (5 kwh's for 4 hours). Broadbit Sodium Battery more energy, quicker charge time, production process is faster.
Potassium-Oxygen Batteries that last longer.
Battery Electric Vehicle is a type of Electric Vehicle (EV) that uses chemical energy stored in rechargeable battery packs. BEVs use electric motors and motor controllers instead of internal combustion engines (ICEs) for propulsion. They derive all power from battery packs and thus have no internal combustion engine, fuel cell, or fuel tank. BEVs include bicycles, scooters, skateboards, rail cars, watercraft, forklifts, buses, trucks and cars.
Battery Technology
Battery Regulator refer to techniques that maximize the capacity of a battery pack with multiple cells in series to make all of its energy available for use and increase the battery's longevity. A battery balancer or battery regulator is a device in a battery pack that performs battery balancing. Balancers are often found in lithium-ion battery packs for cell phones and laptop computers. They can also be found in battery electric vehicle battery packs.
Zinc Carbon Battery is a dry cell battery that delivers a potential of 1.5 volts between a zinc metal electrode and a carbon rod from an electrochemical reaction between zinc and manganese dioxide mediated by a suitable electrolyte.
Zinc Bromine Battery is a type of hybrid flow battery. A solution of zinc bromide is stored in two tanks. When the battery is charged or discharged the solutions (electrolytes) are pumped through a reactor stack and back into the tanks. One tank is used to store the electrolyte for the positive electrode reactions and the other for the negative.
Zinc-Ion Battery that costs half the price of current lithium-ion batteries - Waterloo chemists develop promising cheap, sustainable battery for grid energy storage.
Aerographite is a synthetic foam consisting of a porous interconnected network of tubular carbon.
Aluminum Battery 1 Minute Charging (7,000 charge cycles without capacity degrading. Stable and safe)
Aluminum-Ion Battery Stanford (youtube)
A new concept could make more environmentally friendly batteries possible. A new concept for an aluminium battery has twice the energy density as previous versions, is made of abundant materials, and could lead to reduced production costs and environmental impact. The idea has potential for large scale applications, including storage of solar and wind energy.
Nano-Crystals - Carbon Nanotube
Nanowire Battery uses nanowires to increase the surface area of one or both of its electrodes. Some designs (silicon, germanium and transition metal oxides), variations of the lithium-ion battery have been announced, although none are commercially available. All of the concepts replace the traditional graphite anode and could improve battery performance.
100k Cycles and Beyond: Extraordinary Cycle Stability for MnO2 Nanowires Imparted by a Gel Electrolyte.
New Anode Material Set to Boost Lithium-ion Battery Capacity hybrid anode using silicon-nanolayer-embedded graphite/carbon.
Food waste could store solar and wind energy
Next-generation smartphone battery inspired by the gut lithium-sulphur battery could have five times the energy density of a typical lithium-ion battery. Advanced Lithium–Sulfur Batteries Enabled by a Bio-Inspired Polysulfide Adsorptive Brush.
Flow Battery
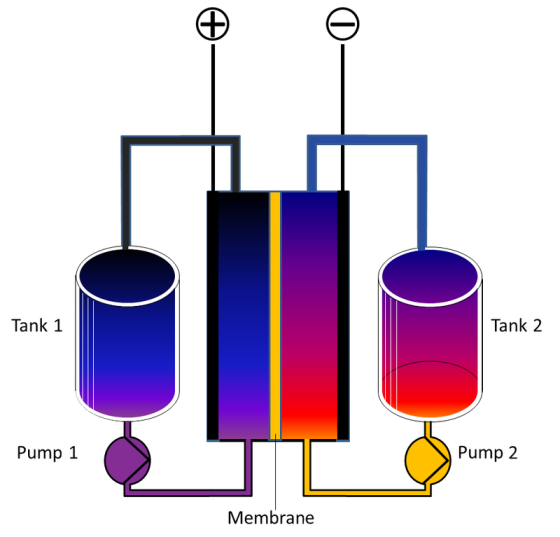 Flow Battery is a
type of rechargeable battery where rechargeability is provided by two
chemical components dissolved in liquids contained within the system and
separated by a membrane. Ion exchange (providing flow of electric current)
occurs through the membrane while both liquids circulate in their own
respective space. Cell voltage is chemically determined by the Nernst
equation and ranges, in practical applications, from 1.0 to 2.2 volts. The
performance of these devices is governed by the considerations of
electrochemical engineering. A flow battery is technically akin both to a
fuel cell and an electrochemical accumulator cell (electrochemical
reversibility). While it has technical advantages such as potentially
separable liquid tanks and near unlimited longevity over most conventional rechargeables, current implementations are comparatively less powerful and
require more sophisticated electronics. The energy capacity is a function
of the electrolyte volume (amount of liquid electrolyte) and the power a
function of the surface area of the electrodes.
Flow Battery is a
type of rechargeable battery where rechargeability is provided by two
chemical components dissolved in liquids contained within the system and
separated by a membrane. Ion exchange (providing flow of electric current)
occurs through the membrane while both liquids circulate in their own
respective space. Cell voltage is chemically determined by the Nernst
equation and ranges, in practical applications, from 1.0 to 2.2 volts. The
performance of these devices is governed by the considerations of
electrochemical engineering. A flow battery is technically akin both to a
fuel cell and an electrochemical accumulator cell (electrochemical
reversibility). While it has technical advantages such as potentially
separable liquid tanks and near unlimited longevity over most conventional rechargeables, current implementations are comparatively less powerful and
require more sophisticated electronics. The energy capacity is a function
of the electrolyte volume (amount of liquid electrolyte) and the power a
function of the surface area of the electrodes. Flow Battery (youtube)
Long-Lasting Flow Battery could Run for more than a Decade with Minimum Upkeep. Battery stores energy in nontoxic, noncorrosive aqueous solutions.
New Battery Material improves Flow Batteries. The material consists of carefully structured molecules designed to be particularly electrochemically stable in order to prevent the battery from losing energy to unwanted reactions. Nonaqueous redox flow.
Organic Mega Flow Battery transcends lifetime, voltage thresholds. Dubbed 'Methuselah', new molecule outlives previous chemistries. Harvard Engineers Organic Mega Flow Battery (youtube).
Organic Redox Flow Batteries - The true path to grid scale energy storage? (youtube)
Scientists have designed an affordable 'flow battery' membrane that could accelerate renewable energy for the electrical grid. Flow battery stores electricity in tanks of liquid electrolyte.
New Battery could store wind and solar electricity affordably and at room temperature. A new type of flow battery that involves a liquid metal more than doubled the maximum voltage of conventional flow batteries and could lead to affordable storage of renewable power.
Salt Water Battery
Salt Water Battery employs a concentrated saline solution as its electrolyte. They are nonflammable and more easily recycled than batteries that employ toxic and/or flammable materials. Aquion Energy.
Molten Salt Battery are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional "use once" thermal batteries can be stored in their solid state at room-temperature for long periods of time before being activated by heating. Rechargeable liquid metal batteries are used for electric vehicles and potentially also for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.
Sodium-Ion Batteries. Sodium, as the sixth most abundant element in the earth’s crust. Solid State.
Thin Layers of Water Hold Promise for the Energy Storage of the Future
Energy Storage
Silicon Air Battery is based on electrodes of oxygen and silicon. Such batteries can be lightweight, with a high tolerance for both extremely dry conditions and high humidity. Like other anode-air batteries, in particular metal-air batteries, silicon–air batteries rely on atmospheric oxygen for their cathodes; they accordingly do not include any cathodes in their structures, and this permits economies in cost and weight.
Aqueous Hybrid Ion Battery uses sodium ions of saltwater as its electricity-carrying electrolyte. Low cost.
Highly Stretchable Aqueous Batteries is a bioinspired Jabuticaba-like hybrid carbon/polymer (HCP) composite that was developed into a stretchable current collector using a simple and cost-effective solution process. Using the HCP composite as a stretchable current collector, the research team has, for the first time, developed a highly stretchable rechargeable lithium-ion battery (ARLB) based on aqueous electrolytes.
Ceramatec Deep Storage Battery (PDF)
Cryogenic Energy Storage is the use of low temperature (cryogenic) liquids such as liquid air or liquid nitrogen as energy storage. Both cryogens have been used to power cars. The inventor Peter Dearman initially developed a liquid air car, and then used the technology he developed for grid energy storage. The technology is being piloted at a UK power station. Highview power (liquid air battery).
Liquid Metal Battery (video)
Liquidmetal a series of amorphous metal alloys with a number of desirable material features, including high tensile strength, excellent corrosion resistance, very high coefficient of restitution and excellent anti-wearing characteristics, while also being able to be heat-formed in processes similar to thermoplastics. Despite the name, they are not liquid at room temperature. New concept turns battery technology upside-down.
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (Group 2, or alkaline earth metals) of the periodic table: all Group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
Researchers Report Breakthrough in Magnesium Batteries. Nanostructured Cathode, Understanding of New Electrolyte Lead to Greater Efficiency.
Lean Electrolyte Design is a game-changer for Magnesium Batteries. Chloride-free electrolyte and organic cathode boosted energy density, stability.
Powerful Battery Created. Metal-oxide magnesium battery cathode material with higher density of energy storage on top of transformative advances in safety, cost and performance in comparison to their ubiquitous lithium-ion (Li-ion) counterparts.
Antimony is a lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name, kohl.
Bio-Battery
Biobattery is an energy storing device that is powered by organic compounds, usually being glucose, such as the glucose in human blood. When enzymes in human bodies break down glucose, several electrons and protons are released. Therefore, by using enzymes to break down glucose, bio-batteries directly receive energy from glucose. These batteries then store this energy for later use. This concept is almost identical to how both plants and many animals obtain energy. Although the batteries are still being tested before being commercially sold, several research teams and engineers are working to further advance the development of these batteries. Electric Nature - Zero Point Batteries.
Purple Bacteria 'Batteries' turn Sewage into Clean Energy. Purple Phototrophic Bacteria -- which can store energy from light -- when supplied with an electric current can recover near to 100 percent of carbon from any type of organic waste, while generating hydrogen gas for use as fuel. Waste Energy.
Electricity-conducting bacteria yield secret to tiny batteries, big medical advances.
Stretchable Battery made entirely out of Fabric. New microbial fuel cell could be integrated into wearable electronics.
Bio-Electro Chemical Reactor are a type of bioreactor where bioelectrochemical processes can take place. They are used in bioelectrochemical syntheses, environmental remediation and electrochemical energy conversion. Examples of bioelectrochemical reactors include microbial electrolysis cells, microbial fuel cells and enzymatic biofuel cells and electrolysis cells, microbial electrosynthesis cells, and biobatteries. This bioreactor is divided in two parts: The anode, where the oxidation reaction takes place; And the cathode, where the reduction occurs.
Human Electromagnetic Field - Human Energy - Life Force
Biomorphic batteries could provide 72 times more energy for robots. Like biological fat reserves store energy in animals, a new rechargeable zinc battery integrates into the structure of a robot to provide much more energy, researchers have shown.
Electrochemical Cell is a device capable of either generating electrical energy from chemical reactions or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt cell meant for consumer use. This type of device is known as a single Galvanic cell. A battery consists of two or more cells, connected in either parallel or series pattern.
Earth Battery is a pair of electrodes made of two dissimilar metals, such as iron and copper, which are buried in the soil or immersed in the sea. Earth batteries act as water activated batteries and if the plates are sufficiently far apart, they can tap telluric currents. Earth batteries are sometimes referred to as telluric power sources and telluric generators.
Magnetite is a mineral and one of the main iron ores. With the chemical formula Fe3O4, it is one of the oxides of iron. Magnetite is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. It is the most magnetic of all the naturally-occurring minerals on Earth. Naturally-magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism. Today it is mined as iron ore.
Fuel Cells (energy)
Proton Battery that's researchable. Proton battery combines the best aspects of hydrogen Fuel Cells and battery-based electrical power. The latest version combines a carbon electrode for solid-state storage of Hydrogen with a reversible fuel cell to provide an integrated rechargeable unit. During charging, protons produced by water splitting in a reversible fuel cell are conducted through the cell membrane and directly bond with the storage material with the aid of electrons supplied by the applied voltage, without forming hydrogen gas. In electricity supply mode this process is reversed; hydrogen atoms are released from the storage and lose an electron to become protons once again. These protons then pass back through the cell membrane where they combine with oxygen and electrons from the external circuit to re-form water. A major potential advantage of the proton battery is much higher energy efficiency than conventional hydrogen systems, making it comparable to lithium ion batteries. The losses associated with hydrogen gas evolution and splitting back into protons are eliminated.
Bacteria-Powered Battery on single sheet of paper
Water Battery charging water by means of a mini water bridge.
Memory Effect is an effect observed in nickel-cadmium and nickel–metal hydride rechargeable batteries that causes them to hold less charge.
Inexpensive Organic Material Gives Safe Batteries a Longer Life. Quinones -- an inexpensive, earth-abundant and easily recyclable material -- to create stable anode composites for any aqueous rechargeable battery.
Quinone represent a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The class includes some heterocyclic compounds. The prototypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply 'quinone' (thus the name of the class). Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.
Electricity Knowledge
SAM L21 32-bit ARM Microcontroller
Superconducting Magnetic Energy Storage Storing Energy in Magnets.
Vanadium Redox Battery
Vanadium is a hard, silvery grey, ductile, and malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation) stabilizes the free metal somewhat against further oxidation.
Battery Stuff - Buffalo Grid
Battery Made from Wood
Nanocellulose and Conductive Polymer
Potato Batteries
Battery Inspired by Vitamins
IV and cellular fluids power Flexible Batteries. Researchers have engineered bendable batteries that can run on body-inspired liquids such as normal IV saline solution and cell-culture medium.
Li-CO2 Electrochemistry: A New Strategy for CO2 Fixation and Energy Storage
New Battery Gobbles up Carbon Dioxide. Lithium-based battery could make use of greenhouse gas before it ever gets into the atmosphere. Researchers are also investigating the possibility of developing a continuous-operation version of the process, which would use a steady stream of carbon dioxide under pressure with the amine material, rather than a preloaded supply the material, thus allowing it to deliver a steady power output as long as the battery is supplied with carbon dioxide. Ultimately, they hope to make this into an integrated system that will carry out both the capture of carbon dioxide from a power plant's emissions stream, and its conversion into an electrochemical material that could then be used in batteries.
NEI Corporation - Saft Batteries - Cal Charge
Portable Wall Outlet
Battery University is a free educational website offering hands-on battery information to engineers, educators, media, students and battery users alike. The tutorials evaluate the advantages and limitations of battery chemistries, advise on best battery choice and suggest ways to extend battery life.
Why do rechargeable batteries measure a higher charge level in cold temperature when they are actually low on power?
Battery Space specialized in all kinds of rechargeable batteries.
Diamond-Age of Power Generation as Nuclear Batteries Developed. Diamond Nuclear-Powered Battery uses nuclear waste to generate electricity in a nuclear-powered battery. A team of physicists and chemists from the University of Bristol have grown a man-made diamond that, when placed in a radioactive field, is able to generate a small electrical current. The development could solve some of the problems of nuclear waste, clean electricity generation and battery life.
Superatoms could make for Better Batteries combinations of atoms that can mimic the properties of more than one group of elements of the periodic table. These superatoms could be used to create new materials.
A new battery concept based on fluoride ions may increase battery lifespans. Fluoride batteries can have a higher energy density, which means that they may last longer -- up to eight times longer than batteries in use todayImagine not having to charge your phone or laptop for weeks. The key to making the fluoride batteries work in a liquid rather than a solid state turned out to be an electrolyte liquid called bis(2,2,2-trifluoroethyl)ether, or BTFE. This solvent is what helps keep the fluoride ion stable so that it can shuttle electrons back and forth in the battery.
Next-Generation Batteries. Engineers build full lithium-ion batteries with silicon anodes and an alumina layer to protect cathodes from degrading. By limiting their energy density, the batteries promise excellent stability for transportation and grid storage use.
Scientists use an industrial laser to turn adhesive tape into a component for safer, anode-free lithium metal batteries. The idea of using tape came from previous attempts to produce free-standing films of laser-induced graphene. Unlike pure polyimide films, the tape produced not only laser-induced graphene from the polyimide backing but also a translucent film where the adhesive had been. The layer formed when they stuck the tape to a copper current collector and lased it multiple times to quickly raise its temperature to 2,300 Kelvin (3,680 degrees Fahrenheit). That generated a porous coating composed primarily of silicon and oxygen, combined with a small amount of carbon in the form of graphene.
Battery Recycling
Battery Recycling is a recycling activity that aims to reduce the number of batteries being disposed as municipal solid waste. Batteries contain a number of heavy metals and toxic chemicals and disposing them by the same process as regular trash has raised concerns over soil contamination and water pollution. Closed Loop Recycling.
Battery Recycling - Battery University - Battery Solutions
Second Life of Lithium-Ion Batteries. Lithium-ion batteries are best suited for second-life usage for power storage over other types of batteries because when their useful life for electric vehicles is over they still retain 80 percent storage capacity for years. So these batteries second life performance can be used to Power Laptop Computers, LED Lights and other low power devices before they degrade. Repurpose.
New polymer material may help batteries become self-healing, recyclable. Engineers have developed a solid polymer-based electrolyte that can self-heal after damage -- and the material can also be recycled without the use of harsh chemicals or high temperatures.
Hydrometallurgy is a method for obtaining metals from their ores. It is a technique within the field of extractive metallurgy involving the use of aqueous chemistry for the recovery of metals from ores, concentrates, and recycled or residual materials. Metal chemical processing techniques that complement hydrometallurgy are pyrometallurgy, vapour metallurgy and molten salt electrometallurgy. Hydrometallurgy is typically divided into three general areas: Leaching. Solution concentration and purification. Metal or metal compound recovery.
Li-Cycle advanced lithium-ion battery recycling technology.
Faraday Institution is the UK’s independent institute for electrochemical energy storage science and technology, supporting research, training, and analysis.
Dismantle is to take something apart or tear it down into pieces.
Disassemble is to take something apart.
Making Batteries From Waste Glass Bottles. UCR researchers are turning glass bottles into high performance lithium-ion batteries for electric vehicles and personal electronics.
Rare Earth Elements - E-Waste
Recycling Nickel-Metal Hydride Batteries
Efficient Perovskite Solar Cells from Recycled Car Batteries
Recycling Knowledge
Batteries from Scrap Metal. Direct conversion of rusty stainless steel mesh into stable, low-cost electrodes for potassium-ion batteries.
New 'Blue-Green' solution for Recycling world's Batteries. Materials scientists demonstrate an environmentally friendly solution to remove valuable cobalt and lithium metals from spent lithium-ion batteries. The metals and the eutectic solvent they use to extract them can then be recycled. The solvent, made of commodity products choline chloride and ethylene glycol, extracted more than 90 percent of cobalt from powdered compounds, and a smaller but still significant amount from used batteries.
When you here someone say that batteries cause to much pollution ask them what type of battery and what process are they talking about?
Environmental Websites
Catalyst - Battery Powered Homes (2016) Jonica Newby (youtube 28:41).
Battery-free small devices runs forever. The device harvests energy from the sun and from the users pressing buttons. Researchers designed the system hardware and software from the ground up to be energy aware as well as very energy efficient. They also developed a new technique for storing the system state in non-volatile memory, minimizing overhead and allowing quick restoration when power returns. This eliminates the need to press "save" as seen in traditional platforms.