BK101
Knowledge Base
Cancer
Knowledge and Information about Cancer. Some of the things you should know about Cancer.
Cancer Kills 20,000 People Every Single Day around the World. Almost 2,000 everyday in the U.S. alone. 8 Million People Die Every Year from Cancer Globally. In 1900 only 1 of 20 got cancer, in 2020 it's 1 in 3 get cancer. 57 percent of cancer cases now occur in low and middle-income countries. 65 percent of cancer deaths worldwide occur in these countries. Don't wait till you get cancer to start eating healthier. Cancer worldwide is expected to Rise by 75 % over the next 20 years.
Cancer Screening - Benign - Immune System - Cancer Therapies - Skin Cancer
 In 2012
there were 14.1 million new cases of cancer around the globe. One in two
men and one in three women will be diagnosed with cancer.
In 2013 there will be 1,660,290
new cancer cases and 580,350
cancer deaths projected to occur in the
U.S. In 2016, an estimated 1,685,210 new
cases of cancer will be diagnosed in the United States and 595,690 people
will die from the disease. In 2019, roughly
1.8 million people will be diagnosed with cancer in the United States.
Over
50 children in the age group of one month to 14 years die of cancer every
day in India. The rate of mortality due to
pediatric cancer in India is at 37 per million every year. According to
Cancer Research UK, 54% of men and 48% of women will get cancer at
some point in their lives.
Just 10 cancers in eight organs, the
blood and the
lymphatic system, will account for more than 70 percent of new cancer cases in the United
States this year in 2017, according to estimates from the
American Cancer Society.
In 2012
there were 14.1 million new cases of cancer around the globe. One in two
men and one in three women will be diagnosed with cancer.
In 2013 there will be 1,660,290
new cancer cases and 580,350
cancer deaths projected to occur in the
U.S. In 2016, an estimated 1,685,210 new
cases of cancer will be diagnosed in the United States and 595,690 people
will die from the disease. In 2019, roughly
1.8 million people will be diagnosed with cancer in the United States.
Over
50 children in the age group of one month to 14 years die of cancer every
day in India. The rate of mortality due to
pediatric cancer in India is at 37 per million every year. According to
Cancer Research UK, 54% of men and 48% of women will get cancer at
some point in their lives.
Just 10 cancers in eight organs, the
blood and the
lymphatic system, will account for more than 70 percent of new cancer cases in the United
States this year in 2017, according to estimates from the
American Cancer Society.
An Ounce of Prevention is worth a pound of cure. Most cancers are preventable. As many as 40 percent of cancer cases, and half of cancer deaths, come down to things people could easily change. Smoking causes 80 to 90 percent of lung cancer deaths. So the best cure for cancer is to avoid the things that cause cancer, which is easier said than done.
More than 15.5 million children and adults with a history of cancer were alive on January 1, 2016, in the United States. By January 1, 2026, it is estimated that the population of cancer survivors will increase to 20.3 million: almost 10 million males and 10.3 million females. But many of the deadliest cancers receive the least amount of research funding like lung and liver, are underfunded. Colon, endometrial, liver and bile duct, cervical, ovarian, pancreatic and lung cancers. In developed countries, more than 80% of children suffering with cancer are cured because of significant progress in treatment. List of Cancer Mortality Rates in the United States (wiki) - Body Burden - Epigenetics.
Cancer Drug Spending tops $100 Billion in 2014, up 10% in a Year. Two-thirds of Americans diagnosed with cancer now live at least five years, versus just more than half in 1990. Living long enough to give drug companies more profits. War Profiteers. There is no Profit in the Cure. There is only profit in the Treatment. Despite all the pink ribbons and billions of dollars in research, another 246,000 women will be diagnosed with breast cancer this year. The Number Of Women Dying Of Cancer Could Double By 2030.
Pinkwashing describes the practice of companies connecting their products to breast cancer awareness and fundraising, often while ignoring the ways their products may contribute to environmental cancer through the materials or methods used in production. Green Washing - Front-men.
Epidemiology of Cancer is the study of the factors affecting cancer, as a way to infer possible trends and causes. The study of cancer epidemiology uses epidemiological methods to find the cause of cancer and to identify and develop improved treatments. This area of study must contend with problems of lead time bias and length time bias. Lead time bias is the concept that early diagnosis may artificially inflate the survival statistics of a cancer, without really improving the natural history of the disease. Length bias is the concept that slower growing, more indolent tumors are more likely to be diagnosed by screening tests, but improvements in diagnosing more cases of indolent cancer may not translate into better patient outcomes after the implementation of screening programs. A related concern is overdiagnosis, the tendency of screening tests to diagnose diseases that may not actually impact the patient's longevity. This problem especially applies to prostate cancer and PSA screening. Some cancer researchers have argued that negative cancer clinical trials lack sufficient statistical power to discover a benefit to treatment. This may be due to fewer patients enrolled in the study than originally planned.
Cancer Screening - Early Detection
 Cancer Screening aims to detect cancer before symptoms appear. This may involve blood tests, urine
tests, other tests, or medical imaging. The benefits of screening in terms
of cancer prevention, early detection and subsequent treatment must be
weighed against any harms. 1800-4-CANCER or 1-800-422-6237 - NCI Cancer
Information Service.
Cancer Screening aims to detect cancer before symptoms appear. This may involve blood tests, urine
tests, other tests, or medical imaging. The benefits of screening in terms
of cancer prevention, early detection and subsequent treatment must be
weighed against any harms. 1800-4-CANCER or 1-800-422-6237 - NCI Cancer
Information Service.
Early Cancer Detection (video) Jorge Soto
Test for Pancreatic Cancer (video) - 15-year-old Jack Andraka
New Device Accurately Identifies Cancer in Seconds MasSpec Pen rapidly and accurately detects cancer in humans during surgery, helping improve treatment and reduce the chances of cancer recurrence.
Chip-based optical sensor detects cancer biomarker in urine. Researchers have used a chip-based sensor with an integrated laser to detect very low levels of a cancer protein biomarker in a urine sample. The new technology is more sensitive than other designs and could lead to non-invasive and inexpensive ways to detect molecules that indicate the presence or progression of a disease.
OnCoBlot Blood Serum Test helps identify up to 25 different cancers with a single test and it is 96% accurate?
A new approach to detecting cancer earlier from blood tests. Cancer scientists have combined 'liquid biopsy,' epigenetic alterations and machine learning to develop a blood test to detect and classify cancer at its earliest stages.
Blood test detects over 50 types of cancer, some before symptoms appear. Developed by GRAIL, Inc., of Menlo Park, Calif., the test uses next-generation sequencing to analyze the arrangement of chemical units called methyl groups on the DNA of cancer cells. Adhering to specific sections of DNA, methyl groups help control whether genes are active or inactive. In cancer cells, the placement of methyl groups, or methylation pattern, is often markedly different from that of normal cells -- to the extent that abnormal methylation patterns are even more characteristic of cancer cells than genetic mutations are. When tumor cells die, their DNA, with methyl groups firmly attached, empties into the blood, where it can be analyzed by the new test.
Fecal Immunochemical Test is a screening test for colon cancer. It tests for hidden blood in the stool, which "could" be an early sign of cancer. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. (FIT).
Liquid Biopsies is a Blood test to detect Circulating Tumor Cells, which are cells that have shed into the vasculature or lymphatics from a primary tumor and are carried around the body in the circulation. A simple new blood test that can catch cancer early.
Blood Test that detects Breast Cancer
I.D. Verification for Cancer. Tiny devices made of DNA detect cancer with fewer false alarms. DNA circuits could help ensure that cancer screens and therapies zero in on the right culprits. A new cancer-detecting tool uses tiny circuits made of DNA to identify cancer cells by the molecular signature on their surface. The circuits work by attaching to the outside of a cell and analyzing it for proteins that are more abundant on some cell types than others. The devices distinguish cell types with higher specificity than previous methods, giving researchers hope their work could improve diagnosis, or give cancer therapies better aim.
Cancer App - Cloud4Cancer Breast Cancer Detection
Freenome aims to diagnose cancer from blood samples. It examines DNA fragments in the bloodstream that are spewed out by cells as they die. Using deep learning, it asks computers to find correlations between cell-free DNA and some cancers.
TINY cancer detection device proves effective in Uganda testing. About half the size of a lunch box, the Tiny Isothermal Nucleic acid quantification sYstem (or TINY) has shown promise as a point-of-care detector of Kaposi sarcoma-associated herpesvirus (KSHV) in resource-limited settings such as sub-Saharan Africa.
Nanowire Device to Detect Cancer with a Urine Test. After introducing a urine sample onto the device, extracellular vesicles are captured by a nanowire substrate via electrostatic forces. MicroRNAs can then be directly extracted from the substrate. Cells communicate with each other through a number of different mechanisms. In animals, for example, predatory threats can drive the release of norepinephrine, a hormone that travels through the bloodstream and triggers heart and muscle cells to initiate a "fight-or-flight" response. A far less familiar mode of cellular transport is the extracellular vesicle. EVs can be thought of as small "chunks" of a cell that are able to pinch off and circulate throughout the body to deliver messenger cargo to other cells. These messengers have become increasingly recognized as crucial mediators of cell-to-cell communication.
Using the Swimming Power of Sperm to Ferry a Cancer Drug directly to a Cervical Tumors - Tiny Machines
Cancer-Fighting Nanorobots programmed to seek and destroy and shrink tumors by cutting off their blood supply. Study shows first applications of DNA origami for Nanomedicine.
Olfactory Cells may act as Trojan Horse to carry Anticancer Therapy to Deadly Brain Tumors. Cells that protect newly generated neurons can be genetically engineered to activate anticancer drug, reduce tumor growth and improve survival in mouse model.
Induced Pluripotent Stem Cells could serve as Cancer Vaccine. Stem Cells.
Cellular Process that Stops Cancer before it starts. Cellular recycling process, thought to fuel cancer's growth, can actually prevent it.
Gene Fusion shifts cell activity into high gear, causing some cancer. Researchers have discovered that a common fusion of two adjacent genes can cause cancer by kicking mitochondria into overdrive, increasing the amount of fuel available for rampant cell growth.
Small Molecule plays a big role in Reducing Cancer’s Spread. Molecule that helps regulate gene expression plays a big role in keeping us safe from the machinations of cancer. In human lung cancer cells, they have shown low levels of the microRNA, miR-125a-5p, which enables the death of aberrant cells like cancer cells, correlates with high levels of the protein TIMP-1, which is already associated with a poor prognosis in patients with cancer. Conversely, when they decrease TIMP-1 levels in these highly lethal cancer cells, tumor spread goes down while rates of cell death go up along with expression of miR-125a-5p.
Sneaky Cancer Cells Stopped in their Tracks. A new study by biomedical engineers shows how they stopped cancer cells from moving and spreading, even when the cells changed their movements. After targeting the "motors" that generate forces in cancer cells to move, the cancer cells switch to a dendritic or "flowing" response to follow pathways in tumors that drive cell migration and promote spreading of the cancer. By using these controlled network microenvironments, researchers were able to test hundreds of cell movement events in hours compared to one or two in the same time frame by imaging a tumor.
Molecular Signal helps Cancer Cells dodge death (youtube) - Cancer cells send a “don’t eat me” signal to stop the immune system from attacking them comes from a protein called CD24. When the researchers blocked the CD24 signal in mice implanted with human cancers, they found this allowed the immune cells, called macrophages, to attack the cancer cells.
Scientists find a cellular process that stops cancer before it starts. Cellular recycling process, thought to fuel cancer's growth, can actually prevent it.
Tailoring cancer treatments to individual patients. Supercomputers help researchers design cancer models and predict treatments outcomes based on patient-specific conditions.
Testing Cells for Cancer Drug Resistance. Biophysicists have demonstrated that Raman microscopy can be used to detect the resistance of tumor cells to cancer drugs. Unlike conventional approaches, this method does not require any antibodies or markers. It detects the response of cells to administered drugs and therefore could determine the effect of drugs in preclinical studies.
Stopping the spread of Glioblastoma with a new drug AMD3100. Glioblastoma is the deadliest form of Brain Cancer, this fluid has a much higher pressure, causing it to move fast and forcing cancer cells to spread. Convection Enhanced Delivery, caused Glioma Cells to invade the rest of the brain.
Pediatric Leukemia 'Super Drug' could be developed in the coming years.
Protein widely known to Fight Tumors also Boosts Cancer Growth. PUMA WTp53 Protein works inside the cell's mitochondria to switch energy production processes and stimulate cancer growth.
Bacteria can Promote Lung Tumor Development. Antibiotics or anti-inflammatory drugs may help combat lung cancer.
Bacteria in Soil is capable of manufacturing streptozotocin, an antibiotic compound that is also an important treatment for certain types of pancreatic cancer. The compound chemical structure known as a nitrosamineis produced through an enzymatic pathway, reveals the novel chemistry that drives the process. Soil.
Researchers develop prostate cancer prediction tool that has unmatched accuracy using machine-learning framework that distinguishes between low- and high-risk prostate cancer with more precision than ever before. Prostate cancer is one of the leading causes of cancer death in American men, second only to lung cancer. PI-RADS v2 Scoring.
New cancer-driving mutation in 'dark matter' of the cancer genome. Change in just one letter of DNA code in a gene conserved through generations of evolution can cause multiple types of cancer. A research group has discovered a novel cancer-driving mutation in the vast non-coding regions of the human cancer genome, also known as the 'dark matter' of human cancer DNA. The U1-snRNA mutation was found in patient tumours with certain subtypes of brain cancer, including nearly all of the studied samples from adult patients with sonic hedgehog medulloblastoma. The mutation was also found in samples of chronic lymphocytic leukemia (CLL) -- the most common type of adult leukemia -- and hepatocellular carcinoma -- the most common type of liver cancer.
A hidden route for fatty acids can make cancers resistant to therapy.
Simply Shining Light on 'Dinosaur Metal' compound kills cancer cells. A new compound based on iridium, a rare metal which landed in the Gulf of Mexico 66 million years ago, hooked onto albumin, a protein in blood, can attack the nucleus of cancerous cells when switched on by light, researchers have found.
Can solar technology kill cancer cells? Scientists have revealed a new way to detect and attack cancer cells using technology traditionally reserved for solar power. The results showcases dramatic improvements in light-activated fluorescent dyes for disease diagnosis, image-guided surgery and site-specific tumor treatment. Fluorescent dyes used for therapeutics and diagnostics, aka theranostics. By optoelectronically tuning organic salt nanoparticles used as theranostics, the Lunts were able to control them in a range of cancer studies. Coaxing the nanoparticles into the nontoxic zone resulted in enhanced imaging, while pushing them into the phototoxic -- or light-activated -- range produced effective on-site tumor treatment. The key was learning to control the electronics of their photoactive molecules independently from their optical properties and then making the leap to apply this understanding in a new way to a seemingly unrelated field. Richard had recently discovered the ability to electronically tune these salts from his work in converting photovoltaics into solar glass. Sophia had long studied metabolic pathways unique to cancer cells. It was when the Lunts were discussing solar glass during a walk that they made the connection: Molecules active in the solar cells might also be used to more effectively target and kill cancer cells.
Artificial intelligence tracks down leukemia. Largest metastudy to date on acute myeloid leukemia.
How mysterious circular DNA causes cancer in children. Mysterious rings of DNA known as extrachromosomal circular DNA can contribute to cancer development in children.
Extrachromosomal Circular DNA are circular DNA found in human, plant and animal cells in addition to chromosomal DNA. eccDNA originate from chromosomal DNA and can be from 50 base pairs to approximately one thousand base pairs in length. eccDNAs that are 200-400 bp in size were recently recoined as microDNA, to draw attention to their size. eccDNA, ecDNA or micro DNA are not to be confused with circular RNA or circRNA. Large extrachromosomal DNA (denoted originally as double minutes, and now re-branded as ecDNA) were discovered in 1970s in human cancer cells by Jerome Vinograd at Caltech and Robert Schimke at Stanford (and doctoral student Fred Alt). This type of ecDNA has unique characteristics: (1) ecDNA found in cancer cells contain one or more genes that confer a selective advantage, (2) they are larger in size than eccDNA, generally ranging in size from 100 Kb to 1-3 Mb and beyond, (3) they are visible by light microscopy. These large ecDNA molecules have been found in the nuclei of human cancer cells and are shown to carry many copies of driver oncogenes which are transcribed in tumour cells. Based on this evidence it is thought that these ecDNA contributes to cancer growth and resistance to chemotherapy drugs.
Most people no longer produce Siglec-12 protein, but some of those who do are at twice the risk for advanced cancer. The primate SIGLEC12 gene encodes one of the CD33-related Siglec family of signaling molecules in immune cells. We had previously reported that this gene harbors a human-specific missense mutation of the codon for an Arg residue required for sialic acid recognition. Here we show that this R122C mutation of the Siglec-XII protein is fixed in the human population, i.e. it occurred prior to the origin of modern humans. Additional mutations have since completely inactivated the SIGLEC12 gene in some but not all humans. The most common inactivating mutation with a global allele frequency of 58% is a single nucleotide frameshift that markedly shortens the open reading frame. Unlike other CD33-related Siglecs that are primarily found on immune cells, we found that Siglec-XII protein is expressed not only on some macrophages but also on various epithelial cell surfaces in humans and chimpanzees. We also found expression on certain human prostate epithelial carcinomas and carcinoma cell lines. This expression correlates with the presence of the nonframeshifted, intact SIGLEC12 allele. Although SIGLEC12 allele status did not predict prostate carcinoma incidence, restoration of expression in a prostate carcinoma cell line homozygous for the frameshift mutation induced altered regulation of several genes associated with carcinoma progression. These stably transfected Siglec-XII-expressing prostate cancer cells also showed enhanced growth in nude mice. Finally, monoclonal antibodies against the protein were internalized by Siglec-XII-expressing prostate carcinoma cells, allowing targeting of a toxin to such cells. Polymorphic expression of Siglec-XII in humans thus has implications for prostate cancer biology and therapeutics.
Dozens of non-oncology drugs can kill cancer cells. Researchers tested approximately 4,518 drug compounds on 578 human cancer cell lines and found nearly 50 that have previously unrecognized anti-cancer activity. These drugs have been used to treat conditions such as diabetes, inflammation, alcoholism, and even arthritis in dogs. The findings suggest a possible way to accelerate the development of new cancer drugs or repurpose existing drugs to treat cancer. The researchers tested all the compounds in the Drug Repurposing Hub on 578 human cancer cell lines from the Broad's Cancer Cell Line Encyclopedia (CCLE). Using a molecular barcoding method known as PRISM, which was developed in the Golub lab, the researchers tagged each cell line with a DNA barcode, allowing them to pool several cell lines together in each dish and more quickly conduct a larger experiment. The team then exposed each pool of barcoded cells to a single compound from the repurposing library, and measured the survival rate of the cancer cells. Nearly a dozen non-oncology drugs killed cancer cells that express a protein called PDE3A by stabilizing the interaction between PDE3A and another protein called SLFN12 -- a previously unknown mechanism for some of these drugs. Most of the non-oncology drugs that killed cancer cells in the study did so by interacting with a previously unrecognized molecular target. For example, the anti-inflammatory drug tepoxalin, originally developed for use in people but approved for treating osteoarthritis in dogs, killed cancer cells by hitting an unknown target in cells that overexpress the protein MDR1, which commonly drives resistance to chemotherapy drugs. The researchers were also able to predict whether certain drugs could kill each cell line by looking at the cell line's genomic features, such as mutations and methylation levels, which were included in the CCLE database. This suggests that these features could one day be used as biomarkers to identify patients who will most likely benefit from certain drugs. For example, the alcohol dependence drug disulfiram (Antabuse) killed cell lines carrying mutations that cause depletion of metallothionein proteins. Compounds containing vanadium, originally developed to treat diabetes, killed cancer cells that expressed the sulfate transporter SLC26A2.
Researchers destroy cancer cells with ultrasound treatment. Technique combines ultrasound application and tumor-targeted microbubbles that attach to cancer cells and explode.
A 'switch' that turns autoimmunity drugs into powerful anti-cancer treatments. Scientists have discovered a way to transform antibody drugs previously developed to treat autoimmunity into antibodies with powerful anti-cancer activity through a simple molecular 'switch'.
Cancer's hidden vulnerabilities. To fight cancer more effectively, a researcher probes its inner workings for metabolic weakness. Utilizing a technique called Raman spectroscopy in conjunction with its advanced version, stimulated Raman scattering (SRS) microscopy. Raman spectroscopy takes advantage of the natural vibrations that occur in the bonds between the atoms that make up a molecule. In this method, a molecule is bombarded with laser light. As the laser light's photons bounce off the molecule, they gain or lose energy as a result of their interaction with the vibrations in the molecule's bonds. Because each kind of bond in a molecule affects photons in a unique and predictable way, the structure of the molecule can be deduced by how the photons "look" after they bounce off of it. By mapping the distribution of targeted chemical bonds, SRS microscopy then provides imagery of these molecular structures.
Cancer Treatment is So Expensive that 42% of patients deplete their entire life's assets to afford treatment within the first 2 years, according to a new study. Patients faced higher likelihood of asset depletion with worsening cancer, continuing treatment, and increasing age. Some patients with imminently fatal cancer still receive treatment. Patients who died within one month of being newly diagnosed with metastatic cancer in the United States received ineffective surgery, chemotherapy, radiation, and hormonal therapy.
Cancer Terminology
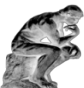
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. Not all tumors are cancerous; benign tumors do not spread to other parts of the body. Possible signs and symptoms include a lump, abnormal bleeding, prolonged cough, unexplained weight loss and a change in bowel movements. While these symptoms may indicate cancer, they may have other causes. Over 100 cancers affect humans. Cancer (gov).
Melanoma is a malignant skin tumor. (mel-eh-noh-ma).
Neoplasm, or Tumor, is an abnormal growth of tissue, and, when it also forms a mass, is commonly referred to as a tumor. This abnormal growth (neoplasia) usually but not always forms a mass. Tumor is an abnormal mass of tissue that can harm organs in the body and cause death by swelling or causing inflammation.
Malignant is a dangerous uncontrolled growth of a tumor that easily spreads like an infection and is poisonous and harmful. Malignancy is the tendency of a medical condition to become progressively worse.
Stage 0 Cancer means there's no cancer, only abnormal cells with the potential to become cancer. This is also called carcinoma in situ.
Stage I Cancer means the cancer is small and only in one area. This is also called early-stage cancer.
Stage II and III Cancer means the cancer is larger and has grown into nearby tissues or lymph nodes.
Stage IV Cancer means the cancer has spread to other parts of your body. It's also called advanced or metastatic cancer.
Benign is harmless and not malignant. (bih-nyn). Benign Tumor is a mass of cells (tumor) that lacks the ability to invade neighboring tissue or metastasize. These characteristics are required for a tumor to be defined as cancerous and therefore benign tumors are non-cancerous.
Metastasis is the spread of a cancer or other disease from one organ or part of the body to another without being directly connected with it. The new occurrences of disease thus generated are referred to as metastases (mets). Cancer occurs after a single cell in a tissue is progressively genetically damaged to produce cells with uncontrolled proliferation. This uncontrolled proliferation by mitosis produces a primary heterogeneic tumour. The cells which constitute the tumor eventually undergo metaplasia, followed by dysplasia then anaplasia, resulting in a malignant phenotype. This malignancy allows for invasion into the circulation, followed by invasion to a second site for tumorigenesis. Metastasized is the spread of cancer to other areas in the body by metastasis, which is the development of secondary malignant growths at a distance from a primary site of cancer. Metastatic Cancer.
Scientists identify protein that promotes brain metastasis. The protein, CEMIP, will now be a focus of efforts to predict, prevent and treat brain metastases, which are a frequent cause of cancer deaths. Cell migration-inducing and hyaluronan-binding protein or CEMIP, formerly known as KIAA1199, is a protein that in humans is encoded by the CEMIP gene. CEMIP has been shown to bind hyaluronic acid and catalyze its depolymerization independently of CD44 and hyaluronidases. Such function has been also been validated in mice.
Infiltration in medical is the diffusion or accumulation (in a tissue or cells) of foreign substances or in amounts in excess of the normal. The material collected in those tissues or cells is called infiltrate. As part of a disease process, infiltration is sometimes used to define the invasion of cancer cells into the underlying matrix or the blood vessels. Similarly, the term may describe the deposition of amyloid protein. During leukocyte extravasation, white blood cells move in response to cytokines from within the blood, into the diseased or infected tissues, usually in the same direction as a chemical gradient, in a process called chemotaxis. The presence of lymphocytes in tissue in greater than normal numbers is likewise called infiltration. As part of medical intervention, local anaesthetics may be injected at more than one point so as to infiltrate an area prior to a surgical procedure. However, the term may also apply to unintended iatrogenic leakage of fluids from phlebotomy or intravenous drug delivery procedures, a process also known as extravasation or "tissuing".
Carcinogenesis is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division, in some cancers forming a malignant mass. Cell division is a physiological process that occurs in almost all tissues and under many circumstances. Under normal circumstances, the balance between proliferation and programmed cell death, usually in the form of apoptosis, is maintained by regulation of both processes to ensure the integrity of tissues and organs. (tumorigenesis).
Cell-Free Tumour DNA is tumour DNA circulating freely in the blood of a cancer patient. Analysis of the fraction of mutant-alleles from ctDNA compared to normal-alleles from the patients normal genome provides opportunities for minimally-invasive cancer diagnosis, prognosis and tumour monitoring. ctDNA originates from dying tumour cells and can be present in a wide range of cancers but at varying levels and mutant allele fractions. The ctDNA is highly fragmented to around 170 bp and is cleared rapidly after surgery to remove tumours or chemotherapeutic treatment.
Mitosis is a part of the cell cycle when replicated chromosomes are separated into two new nuclei. In general, mitosis (division of the nucleus) is preceded by the S stage of interphase (during which the DNA is replicated) and is often accompanied or followed by cytokinesis, which divides the cytoplasm, organelles and cell membrane into two new cells containing roughly equal shares of these cellular components. Inflammation.
Tumour Heterogeneity describes the observation that different tumour cells can show distinct morphological and phenotypic profiles, including cellular morphology, gene expression, metabolism, motility, proliferation, and metastatic potential.
Glioblastoma Multiforme is the most aggressive cancer that begins within the brain.
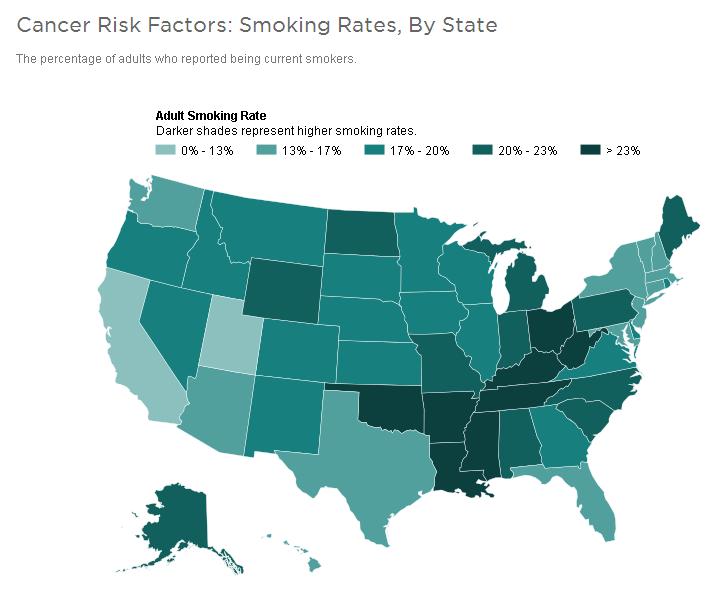 Carcinogen is any substance,
radionuclide, or radiation that is an agent directly involved in causing
cancer. This may be due to the ability to damage the genome or to the
disruption of cellular metabolic processes. Several radioactive substances
are considered carcinogens, but their carcinogenic activity is attributed
to the radiation, for example gamma rays and alpha particles, which they
emit. Common examples of non-radioactive carcinogens are inhaled asbestos,
certain dioxins,
and tobacco smoke. Although
the public generally associates carcinogenicity with
synthetic chemicals,
it is equally likely to arise in both natural and synthetic substances.
Carcinogens are not necessarily immediately toxic, thus their effect can
be insidious.
Carcinogenic is something that is cancer-producing. (kar-sin-oh-jen-ik).
Carcinogen is any substance,
radionuclide, or radiation that is an agent directly involved in causing
cancer. This may be due to the ability to damage the genome or to the
disruption of cellular metabolic processes. Several radioactive substances
are considered carcinogens, but their carcinogenic activity is attributed
to the radiation, for example gamma rays and alpha particles, which they
emit. Common examples of non-radioactive carcinogens are inhaled asbestos,
certain dioxins,
and tobacco smoke. Although
the public generally associates carcinogenicity with
synthetic chemicals,
it is equally likely to arise in both natural and synthetic substances.
Carcinogens are not necessarily immediately toxic, thus their effect can
be insidious.
Carcinogenic is something that is cancer-producing. (kar-sin-oh-jen-ik).List of IARC Group 2B Carcinogens (wiki)
Cellphones and Cancer
Mesothelioma is a rare, aggressive form of cancer that is caused by a person either inhaling or ingesting tiny asbestos fibers.
Oncogene is a gene that has the potential to cause cancer. In tumor cells, they are often mutated or expressed at high levels. Most normal cells will undergo a programmed form of rapid cell death (apoptosis) when critical functions are altered. Activated oncogenes can cause those cells designated for apoptosis to survive and proliferate instead. Most oncogenes require an additional step, such as mutations in another gene, or environmental factors, such as viral infection, to cause cancer. Since the 1970s, dozens of oncogenes have been identified in human cancer. Many cancer drugs target the proteins encoded by oncogenes.
Oncology is a branch of medicine that deals with the prevention, diagnosis and treatment of cancer. A medical professional who practices oncology is an oncologist. The three components which have improved survival in cancer are: Prevention - This is by reduction of risk factors like tobacco and alcohol consumption. Early diagnosis - Screening of common cancers and comprehensive diagnosis and staging. Treatment - Multimodality management by discussion in tumor board and treatment in a comprehensive cancer centre.
Oncology Database
US Oncology
Clinical Oncology
Oncology News
Childhood Cancer Organization
Understanding Cancer
mtDNA mutations increase tumorigenicity in prostate cancer.
Cancer Center
Cancer Research
Epstein-Barr Virus and Cancer (youtube)
The Basis of Oncoimmunology
Cold climate increases Cancer Risk. Populations living in very low temperatures, like in Denmark and Norway, had among the highest incidences of cancer in the world. Cell resistance at low temperatures and at high altitude and diet.
GLOBOCAN-2012 Database
Cytomegalovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and monkeys serve as natural hosts.
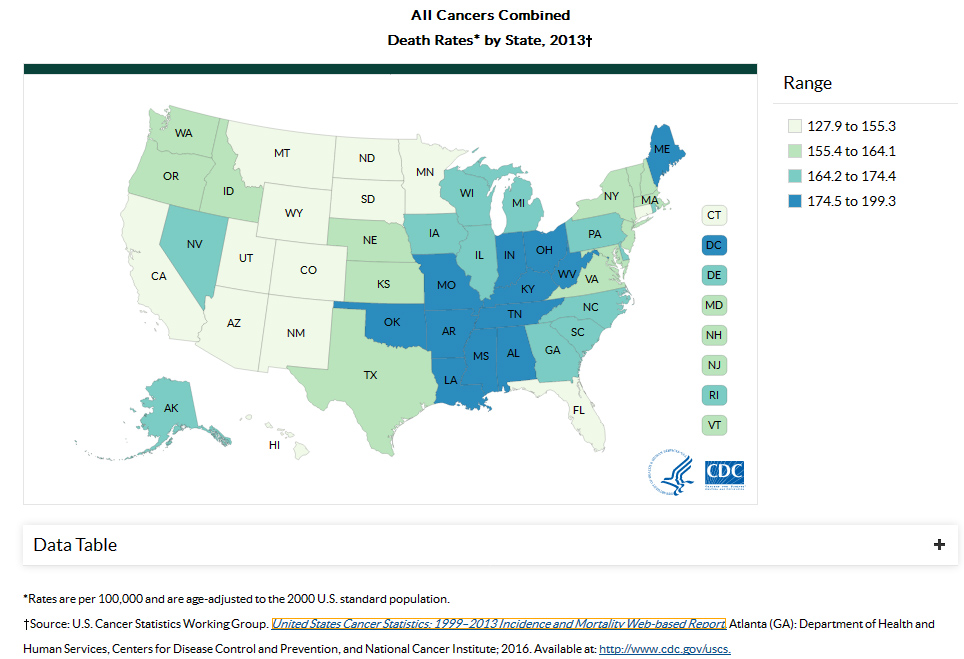 Cancer Clusters is a high number of
cancer cases occurs in a group of people in a particular geographic area
over a limited period of time. Historical examples of work-related cancer
clusters are well documented in the medical literature.
Cancer Clusters is a high number of
cancer cases occurs in a group of people in a particular geographic area
over a limited period of time. Historical examples of work-related cancer
clusters are well documented in the medical literature.Cancer Clusters
Cancer Incidence and Mortality Data (1999–2013)
Cancer Epidemiology, Biomarkers & Prevention is a peer-reviewed medical journal devoted to research in the field of cancer epidemiology. Topics include descriptive, analytical, biochemical, and molecular epidemiology, the use of biomarkers to study the neoplastic and preneoplastic processes in humans, chemoprevention and other types of prevention trials, and the role of behavioral factors in cancer etiology and prevention. Epidemiology of Cancer.
Disease - Pollution - Toxins
Deadly Type of Breast Cancer. Limiting an amino acid called asparagine in laboratory mice who had triple-negative breast cancer could dramatically reduce the ability of the cancer to travel to distant sites in the body.
DNA Repair - Bodies Natural Defenses against Cancer
Tumor Suppressor Gene is a gene that protects a cell from one step on the path to cancer. When this gene mutates to cause a loss or reduction in its function, the cell can progress to cancer, usually in combination with other genetic changes. The loss of these genes may be even more important than proto-oncogene/oncogene activation for the formation of many kinds of human cancer cells. Tumor suppressor genes can be grouped into categories including caretaker genes, gatekeeper genes, and landscaper genes; the classification schemes are evolving as medicine advances, learning from fields including molecular biology, genetics, and epigenetics.
Immune Response - Epigenetics - Error Correcting - Neurogenesis - Regeneration (stem cells) - Extremophiles
Cancer starts from damage in the mitochondria, which could come from exposure to radiation, chemicals or toxins and stress. Cancer ferments sugar and does not need oxygen to grow. Glucose and Glutamine helps to feed cancer and makes it grow. So it's a good idea to lower sugar intake and do some intermittent fasting which sensitizes cancer cells to death, and increases the stress capability of healthy cells. Avoid animal meats and go on a more plant based diet to reduce IGF-1 signalling, which can allow cancer cells to survive under stressors. Supplement lots of broccoli sprouts, sulforaphane in broccoli sprouts has many similar effects to fasting, sensitizing cancer cells to death & increasing stress capability of healthy cells. Eat foods that will help inhibit some of the glutamine intake. Avoid high doses of supplemental antioxidants such as vitamin A or C, which can reduce effects of fasting, exercise and sulforaphane. Avoid all opioids such as morphine, fentanyl, vicodin, oxycodone etc, these increase cancer metastasis. Avoid the ketogenic diet that could be harmful depending on the type of cancer. Optimize her gut microbiome which boosts the immune system in fighting cancer. Increase exercise, has similar effects to fasting and sulforaphane.
Caretaker Gene encode products that stabilize the genome. Fundamentally, mutations in caretaker genes lead to genomic instability. Tumor cells arise from two distinct classes of genomic instability: mutational instability arising from changes in the nucleotide sequence of DNA and chromosomal instability arising from improper rearrangement of chromosomes. In contrast to caretaker genes, gatekeeper genes encode gene products that act to prevent growth of potential cancer cells and prevent accumulation of mutations that directly lead to increased cellular proliferation. The third classification of genes, the landscapers, encode products that, when mutated, contribute to the neoplastic growth of cells by fostering a stromal environment conducive to unregulated cell proliferation.
Neurogenesis - Immune Therapy
Natural Killer Cell
DNA Repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA damage, resulting in as many as 1 million individual molecular lesions per cell per day. Many of these lesions cause structural damage to the DNA molecule and can alter or eliminate the cell's ability to transcribe the gene that the affected DNA encodes. Other lesions induce potentially harmful mutations in the cell's genome, which affect the survival of its daughter cells after it undergoes mitosis. As a consequence, the DNA repair process is constantly active as it responds to damage in the DNA structure. When normal repair processes fail, and when cellular apoptosis does not occur, irreparable DNA damage may occur, including double-strand breaks and DNA crosslinkages (interstrand crosslinks or ICLs). This can eventually lead to malignant tumors, or cancer as per the two hit hypothesis. The rate of DNA repair is dependent on many factors, including the cell type, the age of the cell, and the extracellular environment. A cell that has accumulated a large amount of DNA damage, or one that no longer effectively repairs damage incurred to its DNA, can enter one of three possible states: An irreversible state of dormancy, known as senescence. Cell suicide, also known as apoptosis or programmed cell death. Unregulated cell division, which can lead to the formation of a tumor that is cancerous. The DNA repair ability of a cell is vital to the integrity of its genome and thus to the normal functionality of that organism. Many genes that were initially shown to influence life span have turned out to be involved in DNA damage repair and protection. Teaching Human Cells to Clean House to Delay Aging and Fight Neuro-Degeneration.
Autophagy is the natural, regulated mechanism of the cell that disassembles unnecessary or dysfunctional components. It allows the orderly degradation and recycling of cellular components. Three forms of autophagy are commonly described: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA). In macroautophagy, targeted cytoplasmic constituents are isolated from the rest of the cell within a double-membraned vesicle known as an autophagosome. The
autophagosome eventually fuses with lysosomes and the contents are degraded and recycled. In disease, autophagy has been seen as an adaptive response to stress, which promotes survival, whereas in other cases it appears to promote cell death
and morbidity. In the extreme case of starvation, the breakdown of cellular components promotes cellular survival by maintaining cellular energy levels.
New Repair Mechanism for repairing alcohol-induced DNA Damage. Our DNA is a daily target for a barrage of damage caused by radiation or toxic substances such as alcohol. When alcohol is metabolized, acetaldehyde is formed. Acetaldehyde causes a dangerous kind of DNA damage -- the interstrand crosslink (ICL) -- that sticks together the two strands of the DNA. As a result, it obstructs cell division and protein production. Ultimately, an accumulation of ICL damage may lead to cell death and cancer. Thankfully, every cell in our body possesses a toolkit with which it can repair this type of damage to the DNA. The first line of defense against ICLs caused by acetaldehyde is the ALDH2 enzyme, that largely breaks down acetaldehyde before it causes any harm. However, not everyone profits from this enzyme -- about half of the Asian population, more than 2 billion people worldwide, possess a mutation in the gene coding for this enzyme. Because they are not able to break down acetaldehyde, they are more prone to develop alcohol-related cancer.
TP53 is any isoform of a protein encoded by homologous genes in various organisms, such as TP53 (humans) and Trp53 (mice). This homolog (originally thought to be, and often spoken of as, a single protein) is crucial in multicellular organisms, where it prevents cancer formation, thus, functions as a tumor suppressor. As such, p53 has been described as "the guardian of the genome" because of its role in conserving stability by preventing genome mutation. Hence TP53 is classified as a tumor suppressor gene.(Italics are used to denote the TP53 gene name and distinguish it from the protein it encodes).
P53 Tumor Suppressor Protein
Alternative Lengthening of Telomeres. Telomeres are specialized structures at the ends of the linear chromosomes. Their maintenance is essential for the unlimited proliferation of cells due to the 3'-end erosion, a process intrinsic to the replication of linear chromosomes. Progressive telomere shortening in somatic cells can lead to the induction of senescence or apoptosis, thus acting as a barrier to unlimited proliferation and tumorigenesis, which is the production of a new tumor or tumors.
Tumor Antigen is an antigenic substance produced in tumor cells, i.e., it triggers an immune response in the host. Tumor antigens are useful tumor markers in identifying tumor cells with diagnostic tests and are potential candidates for use in cancer therapy. The field of cancer immunology studies such topics.
The Cancer Genome Atlas is a project, begun in 2005, to catalogue genetic mutations responsible for cancer, using genome sequencing and bioinformatics. TCGA applies high-throughput genome analysis techniques to improve our ability to diagnose, treat, and prevent cancer through a better understanding of the genetic basis of this disease.
Cancer Epigenetics is the study of epigenetic modifications to the genome of cancer cells that do not involve a change in the nucleotide sequence. Epigenetic alterations are as important as genetic mutations in a cell's transformation to cancer, and their manipulation holds great promise for cancer prevention, detection, and therapy.
Bone Morphogenetic Protein are a group of growth factors also known as cytokines and as metabologens. Originally discovered by their ability to induce the formation of bone and cartilage, BMPs are now considered to constitute a group of pivotal morphogenetic signals, orchestrating tissue architecture throughout the body. The important functioning of BMP signals in physiology is emphasized by the multitude of roles for dysregulated BMP signaling in pathological processes. Cancerous disease often involves misregulation of the BMP signaling system. Absence of BMP signaling is, for instance, an important factor in the progression of colon cancer, and conversely, over activation of BMP signaling following reflux-induced esophagitis provokes Barrett's esophagus and is thus instrumental in the development of adenocarcinoma in the proximal portion of the gastrointestinal tract.
A signaling pathway is a group of molecules in a cell that work together to control one or more cell functions. Like a cascade, after the first molecule in a pathway receives a signal, it activates another molecule and so forth until the cell function is carried out.
Cancer Survivor is a person with cancer of any type who is still living. Whether a person becomes a survivor at the time of diagnosis or after completing treatment, whether people who are actively dying are considered survivors, and whether healthy friends and family members of the cancer patient are also considered survivors, varies from group to group. Some people who have been diagnosed with cancer reject the term survivor or disagree with some definitions of it. How many people are cancer survivors depends on the definition used. About 11 million Americans alive today—one in 30 people–are either currently undergoing treatment for cancer or have done so in the past. Currently nearly 65% of adults diagnosed with cancer in the developed world are expected to live at least five years after the cancer is discovered.
Almost 15 percent of Lung Cancer Survivors are still smokers. Prevalence and correlates of smoking and cessation-related behavior among survivors of ten cancers.
While studying the underpinnings of multiple sclerosis, investigators came across important clues for how to treat cancer. Researchers describe an Anti-LAP Antibody that can precisely target regulatory T cells which in turn unleashes the immune system to kill cancer cells. The team reports that the antibody decreased tumor growth in models of melanoma, glioblastoma and colorectal carcinoma, making it an attractive candidate for cancer immunotherapy.
New cancer-driving mutation in 'dark matter' of the cancer genome. Change in just one letter of DNA code in a gene conserved through generations of evolution can cause multiple types of cancer. Non-Coding DNA, which makes up 98 per cent of the genome, is notoriously difficult to study and is often overlooked since it does not code for proteins.
Zombie gene protects against cancer in elephants. Dead gene reborn helps destroy cells with damaged DNA. Master tumor suppressor gene p53. This gene enables humans and elephants to recognize unrepaired DNA damage, a precursor of cancer. Then it causes those damaged cells to die. Pseudogene called leukemia inhibitory factor 6 (LIF6) that had somehow evolved a new on-switch. LIF6, back from the dead, had become a valuable working gene. Its function, when activated by p53, is to respond to damaged DNA by killing the cell. The LIF6 gene makes a protein that goes, quite rapidly, to the mitochondria, the cell's main energy source. That protein pokes holes in the mitochondria, causing the cell to die. "Hence, zombie," said Lynch. "This dead gene came back to life. When it gets turned on by damaged DNA, it kills that cell, quickly. This is beneficial, because it acts in response to genetic mistakes, errors made when the DNA is being repaired. Getting rid of that cell can prevent a subsequent cancer."
Immune System
Immune System is a host defense system comprising many biological structures and processes within an organism that protects against disease. To function properly, an immune system must detect a wide variety of agents, known as pathogens, from viruses to parasitic worms, and distinguish them from the organism's own healthy tissue.
Aids - Autoimmune Disease - Allergies - Immunity - T-Cells - Lymphatic System
Antibody is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen, via the Fab's variable region. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (similarly, analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize its target directly (for example, by inhibiting a part of a microbe that is essential for its invasion and survival). Depending on the antigen, the binding may impede the biological process causing the disease or may activate macrophages to destroy the foreign substance. The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region (located at the base of the "Y"), which contains a conserved glycosylation site involved in these interactions. The production of antibodies is the main function of the humoral immune system. Antibodies are secreted by B cells of the adaptive immune system, mostly by differentiated B cells called plasma cells. Antibodies can occur in two physical forms, a soluble form that is secreted from the cell to be free in the blood plasma, and a membrane-bound form that is attached to the surface of a B cell and is referred to as the B-cell receptor (BCR). The BCR is found only on the surface of B cells and facilitates the activation of these cells and their subsequent differentiation into either antibody factories called plasma cells or memory B cells that will survive in the body and remember that same antigen so the B cells can respond faster upon future exposure. In most cases, interaction of the B cell with a T helper cell is necessary to produce full activation of the B cell and, therefore, antibody generation following antigen binding. Soluble antibodies are released into the blood and tissue fluids, as well as many secretions to continue to survey for invading microorganisms. Antibodies are glycoproteins belonging to the immunoglobulin superfamily. They constitute most of the gamma globulin fraction of the blood proteins. They are typically made of basic structural units—each with two large heavy chains and two small light chains. There are several different types of antibody heavy chains that define the five different types of crystallisable fragments (Fc) that may be attached to the antigen-binding fragments. The five different types of Fc regions allow antibodies to be grouped into five isotypes. Each Fc region of a particular antibody isotype is able to bind to its specific Fc Receptor (except for IgD, which is essentially the BCR), thus allowing the antigen-antibody complex to mediate different roles depending on which FcR it binds. The ability of an antibody to bind to its corresponding FcR is further modulated by the structure of the glycan(s) present at conserved sites within its Fc region. The ability of antibodies to bind to FcRs helps to direct the appropriate immune response for each different type of foreign object they encounter. For example, IgE is responsible for an allergic response consisting of mast cell degranulation and histamine release. IgE's Fab paratope binds to allergic antigen, for example house dust mite particles, while its Fc region binds to Fc receptor ε. The allergen-IgE-FcRε interaction mediates allergic signal transduction to induce conditions such as asthma. Though the general structure of all antibodies is very similar, a small region at the tip of the protein is extremely variable, allowing millions of antibodies with slightly different tip structures, or antigen-binding sites, to exist. This region is known as the hypervariable region. Each of these variants can bind to a different antigen. This enormous diversity of antibody paratopes on the antigen-binding fragments allows the immune system to recognize an equally wide variety of antigens. The large and diverse population of antibody paratope is generated by random recombination events of a set of gene segments that encode different antigen-binding sites (or paratopes), followed by random mutations in this area of the antibody gene, which create further diversity. This recombinational process that produces clonal antibody paratope diversity is called V(D)J or VJ recombination. Basically, the antibody paratope is polygenic, made up of three genes, V, D, and J. Each paratope locus is also polymorphic, such that during antibody production, one allele of V, one of D, and one of J is chosen. These gene segments are then joined together using random genetic recombination to produce the paratope. The regions where the genes are randomly recombined together is the hyper variable region used to recognise different antigens on a clonal basis. Antibody genes also re-organize in a process called class switching that changes the one type of heavy chain Fc fragment to another, creating a different isotype of the antibody that retains the antigen-specific variable region. This allows a single antibody to be used by different types of Fc receptors, expressed on different parts of the immune system.
Immunoglobulins is any of a class of proteins present in the serum and cells of the immune system, which function as antibodies.
Antibiotics (vaccinations)
Monoclonal Antibody are antibodies that are made by identical immune cells that are all clones of a unique parent cell. Monoclonal antibodies can have monovalent affinity, in that they bind to the same epitope (the part of an antigen that is recognized by the antibody).
Macrophage are a type of White Blood Cell, of the immune system, that engulfs and digests cellular debris, foreign substances, microbes, cancer cells, and anything else that does not have the type of proteins specific to healthy body cells on its surface in a process called phagocytosis. These large phagocytes are found in essentially all tissues, where they patrol for potential pathogens by amoeboid movement. They take various forms (with various names) throughout the body (e.g., histiocytes, Kupffer cells, alveolar macrophages, microglia, and others), but all are part of the mononuclear phagocyte system. Besides phagocytosis, they play a critical role in nonspecific defense (innate immunity) and also help initiate specific defense mechanisms (adaptive immunity) by recruiting other immune cells such as lymphocytes. For example, they are important as antigen presenters to T cells. In humans, dysfunctional macrophages cause severe diseases such as chronic granulomatous disease that result in frequent infections.
Neuroimmune System is a system of structures and processes involving the biochemical and electrophysiological interactions between the nervous system and immune system which protect neurons from pathogens. It serves to protect neurons against disease by maintaining selectively permeable barriers (e.g., the blood–brain barrier and blood–cerebrospinal fluid barrier), mediating neuroinflammation and wound healing in damaged neurons, and mobilizing host defenses against pathogens. The neuroimmune system and peripheral immune system are structurally distinct. Unlike the peripheral system, the neuroimmune system is composed primarily of glial cells; among all the hematopoietic cells of the immune system, only mast cells are normally present in the neuroimmune system. However, during a neuroimmune response, certain peripheral immune cells are able to cross various blood or fluid–brain barriers in order to respond to pathogens that have entered the brain. For example, there is evidence that following injury macrophages and T cells of the immune system migrate into the spinal cord. Production of immune cells of the complement system have also been documented as being created directly in the central nervous system.
Humoral Immunity is the aspect of immunity that is mediated by macromolecules found in extracellular fluids such as secreted antibodies, complement proteins, and certain antimicrobial peptides. Humoral immunity is so named because it involves substances found in the humors, or body fluids. It contrasts with cell-mediated immunity. Its aspects involving antibodies are often called antibody-mediated immunity. The study of the molecular and cellular components that form the immune system, including their function and interaction, is the central science of immunology. The immune system is divided into a more primitive innate immune system, and acquired or adaptive immune system of vertebrates, each of which contains humoral and cellular components. Humoral immunity refers to antibody production and the accessory processes that accompany it, including: Th2 activation and cytokine production, germinal center formation and isotype switching, affinity maturation and memory cell generation. It also refers to the effector functions of antibodies, which include pathogen and toxin neutralization, classical complement activation, and opsonin promotion of phagocytosis and pathogen elimination. T-Cells.
Peripheral Immune System is composed of disconnected lymphocyte clones which remain in a resting state unless they are specifically activated by an antigen giving rise to a classical immune response.
Central Immune System is composed of a network of clones which display autonomous activity and integrates antigens into its ongoing regulatory dynamics. Functionally, the PIS is appropriate for reactions to immunizing antigens, whereas the CIS is appropriate for body antigens.
Second generation immune network are unable to account satisfactorily for the CIS/PIS distinction. A third generation immune network Model, incorporating B-T cell co-operation, is able to accommodate both the structural and the functional properties of CIS and PIS in a coherent account, and moreover to explain how the CIS/PIS distinction can be generated by the self-organizing properties of the network. Finally, we emphasize that the difficulty in establishing a productive relationship between theory and experiment is a hallmark of the whole network approach to the immune system, and is perhaps the reason why, at the present time, the immunological community.
Adaptive Immune System is a subsystem of the overall immune system that is composed of highly specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The adaptive immune system is one of the two main immunity strategies found in vertebrates (the other being the innate immune system). Adaptive immunity creates immunological memory after an initial response to a specific pathogen, and leads to an enhanced response to subsequent encounters with that pathogen. This process of acquired immunity is the basis of vaccination. Like the innate system, the adaptive system includes both humoral immunity components and cell-mediated immunity components.
Innate Immune System is an important subsystem of the overall immune system that comprises the cells and mechanisms that defend the host from infection by other organisms. The cells of the innate system recognize and respond to pathogens in a similar way, but, unlike the adaptive immune system, the system does not provide long-lasting immunity to the host. Innate immune systems provide immediate defense against infection, and are found in all classes of plant and animal life. The innate immune system is an evolutionarily older defense strategy, and is the dominant immune system found in plants, fungi, insects, and primitive multicellular organisms. The major functions of the vertebrate innate immune system include: Recruiting immune cells to sites of infection through the production of chemical factors, including specialized chemical mediators called cytokines. Activation of the complement cascade to identify bacteria, activate cells, and promote clearance of antibody complexes or dead cells. Identification and removal of foreign substances present in organs, tissues, blood and lymph, by specialized white blood cells. Activation of the adaptive immune system through a process known as antigen presentation. Acting as a physical and chemical barrier to infectious agents. Virus infection in vertebrates results in two general types of immune response. The first is a rapid-onset "innate" response against the virus, which involves the synthesis of proteins called interferons and the stimulation of "natural killer" lymphocytes. The acquired immune system, with help from the innate system, produces cells (antibodies) to protect your body from a specific invader. These antibodies are developed by cells called B lymphocytes after the body has been exposed to the invader. The cells of both parts of the immune system are made in various organs of the body, including: Adenoids. Two glands located at the back of the nasal passage. Bone marrow. The soft, spongy tissue found in bone cavities. Lymph nodes. Small organs shaped like beans, which are located throughout the body and connect via the lymphatic vessels. Lymphatic vessels. A network of channels throughout the body that carries lymphocytes to the lymphoid organs and bloodstream. Peyer's patches. Lymphoid tissue in the small intestine. Spleen. A fist-sized organ located in the abdominal cavity. Thymus. Two lobes that join in front of the trachea behind the breastbone. Tonsils. Two oval masses in the back of the throat. A special cell of the immune system called a T cell circulates looking for infections. One type of T cell is called a cytotoxic T cell because it kills cells that are infected with viruses with toxic mediators. Cytotoxic T cells have specialized proteins on their surface that help them to recognize virally-infected cells. These proteins are called T cell receptors (TCRs). Each cytotoxic T cell has a TCR that can specifically recognize a particular antigenic peptide bound to an MHC molecule. If the T cell receptor detects a peptide from a virus, it warns its T cell of an infection. The T cell releases cytotoxic factors to kill the infected cell and, therefore, prevent survival of the invading virus.
Immunity is the balanced state of multicellular organisms having adequate biological defenses to fight infection, disease, or other unwanted biological invasion, while having adequate tolerance to avoid allergy, and autoimmune diseases. What is the number of viruses that can be recognized by the immune system?
Immune Response is the immunological response originating from immune system activation by antigens, including immunity to pathogenic microorganisms and its products, as well as autoimmunity to self-antigens allergies, and graft ejections. In this process main cells involved are the T Cells, B cells of lymphocytes, and macrophagea. These cells produce lymphokines that influence the other host cells activities. B cells mature to produce immunoglobulins or antibodies, that react with antigens. At same time, macrophages are processing the antigens into immunogenic units which stimulate B lymphocites to differentiation into antibody secreting plasma cells, stimulating the T cells to realise lymphokines.
Study reveals details of immune defense guidance system. At the beginning of an immune response, a molecule known to mobilize immune cells into the bloodstream, where they home in on infection sites, rapidly shifts position. Researchers say this indirectly amplifies the attack on foreign microbes or the body's own tissues. Past studies had shown that the immune system regulates the concentration of the molecule, sphingosine 1 phosphate or S1P, in order to draw cells to the right locations. The targeted cells have proteins on their surface that are sensitive to levels of this molecule, enabling them to follow the molecule's "trail," researchers say. S1P concentration gradients, for instance, can guide immune T cells to either stay in lymph nodes, connected glands in which these cells mature, or move into blood vessels. For the first time, researchers at NYU Grossman School of Medicine showed in mice experiments that S1P levels in lymph nodes increase as the immune response mounts. Such activation of immune cells can cause inflammation, swelling, and/or death of targeted cells. While past work had shown that S1P is produced by cells attached to lymph nodes, the new study found that monocytes, circulating immune cells, also produced it when mice were infected with a virus. This in turn may influence the migration of T cells, a set of white blood cells that expands rapidly in response to infection.
Immuno-Stimulant are substances (drugs and nutrients) that stimulate the immune system by inducing activation or increasing activity of any of its components. One notable example is the granulocyte macrophage colony-stimulating factor.
Complement System is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promotes inflammation, and attacks the pathogen's plasma membrane. It is part of the innate immune system, which is not adaptable and does not change over the course of an individual's lifetime. It can be recruited and brought into action by the adaptive immune system. The complement system consists of a number of small proteins found in the blood, synthesized by the liver, which circulate as inactive precursors (pro-proteins). When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end result of this complement activation or complement fixation cascade is stimulation of phagocytes to clear foreign and damaged material, inflammation to attract additional phagocytes, and activation of the cell-killing membrane attack complex. Over 30 proteins and protein fragments make up the complement system, including serum proteins, and cell membrane receptors. They account for about 10% of the globulin fraction of blood serum. Three biochemical pathways activate the complement system: the classical complement pathway, the alternative complement pathway, and the lectin pathway.
Boost your Immune System (harvard)
Ebola Virus disables the body's immune defenses by disableing T cells, an important line of immune defense, thus rendering the infected person less able to combat the infection. White blood cells are an important part of our immune system. Lymphopenia happens when the white blood T cell count in the bloodstream is lower than normal -- in fact, the extent of lymphopenia is one of the strongest indicators of how severe the Ebola infection will become.
Scientists stimulate immune system, stop cancer growth.
Evolution of Immune Systems From Viruses and Transposable Elements.
The immune system's fountain of youth. Helping the immune system clear away old cells in aging mice helped restore youthful characteristics.
Human Mast Cells From Adipose Tissue Target and Induce Apoptosis of Breast Cancer Cells. Mast cells (MC) are important immune sentinels found in most tissue and widely recognized for their role as mediators of Type I hypersensitivity. However, they also secrete anti-cancer mediators such as tumor necrosis factor alpha (TNF-α) and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Deciphering the sugar code. Researchers discover vaccine to strengthen the immune system of plants. Like animals and humans, plants possess a kind of immune system. It can e.g. recognize pathogenic fungi by the chitin in their cell walls, triggering disease resistance. Some fungi hide from the immune system by modifying some of the chitin building blocks, converting chitin into chitosan. Researchers now found that plants can react to a certain pattern in this chitosan, stimulating their immune system.
Origins of immune system mapped, opening doors for new cancer immunotherapies. Cell atlas of human thymus could help engineer improved therapeutic T cells. A first cell atlas of the human thymus gland could lead to new immune therapies to treat cancer and autoimmune diseases. Researchers mapped thymus tissue through the human lifespan to understand how it develops and makes vital immune cells called T cells. In the future, this information could help researchers to generate an artificial thymus and engineer improved therapeutic T cells. The atlas could also help scientists understand diseases that affect T cell development such as severe combined immunodeficiency (SCID), and adds to the Human Cell Atlas initiative which is creating a Google map of the entire human body.
Obesity impairs immune cell function, accelerates tumor growth. High-fat diet allows cancer cells to outcompete immune cells for fuel. Cancer cells do so by rewiring their metabolisms to increase fat consumption. Blocking this rewiring enhances anti-tumor immunity. The findings suggest new strategies to target cancer metabolism and improve immunotherapies. Reporting in Cell on Dec. 9, the research team shows that a high-fat diet reduces the numbers and antitumor activity of CD8+ T cells, a critical type of immune cell, inside tumors. This occurs because cancer cells reprogram their metabolism in response to increased fat availability to better gobble up energy-rich fat molecules, depriving T cells of fuel and accelerating tumor growth.
Immunosenescence refers to the gradual deterioration of the immune system brought on by natural age advancement. The adaptive immune system is affected more than the innate immune system. Immunosenescence involves both the host's capacity to respond to infections and the development of long-term immune memory, especially by vaccination. This age-associated immune deficiency is ubiquitous and found in both long- and short-living species as a function of their age relative to life expectancy rather than chronological time. It is considered a major contributory factor to the increased frequency of morbidity and mortality among the elderly. Immunosenescence is not a random deteriorative phenomenon, rather it appears to inversely repeat an evolutionary pattern and most of the parameters affected by immunosenescence appear to be under genetic control. Immunosenescence can also be sometimes envisaged as the result of the continuous challenge of the unavoidable exposure to a variety of antigens such as viruses and bacteria.
Immune Therapy
Immunotherapy is the "treatment of disease by inducing, enhancing, or suppressing an immune response". Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunomodulatory regimens often have fewer side effects than existing drugs, including less potential for creating resistance when treating microbial disease. Cell-based immunotherapies are effective for some cancers. Immune effector cells such as lymphocytes,macrophages, dendritic cells, natural killer cells (NK Cell), cytotoxic T lymphocytes (CTL), etc., work together to defend the body against cancer by targeting abnormal antigens expressed on the surface of tumor cells. Therapies such as granulocyte colony-stimulating factor (G-CSF), interferons, imiquimod and cellular membrane fractions from bacteria are licensed for medical use. Others including IL-2, IL-7, IL-12, various chemokines, synthetic cytosine phosphate-guanosine (CpG) oligodeoxynucleotides and glucans are involved in clinical and preclinical studies. DNA sensor plays critical role in cancer immunotherapy via response to unexpected DNA form.
Living Drug. Most drugs are made from natural or synthetic compounds. Living Drugs consist of fully functional cells that have been selected and often modified to treat specific diseases, such as cancer. Immunotherapy encompasses several types of treatment that marshal the immune system against cancer. Drugs known as cell therapies fall into this category. They are often made by collecting specific sets of cells from a patient’s blood, modifying them to produce or elicit a more vigorous attack on a patient’s cancer cells, and reinfusing them into the patient. Two types of cellular therapy that have recently become available are CAR (chimeric antigen receptor) T cells and therapeutic vaccines. CAR T cells are immune system T cells that have been genetically engineered to latch onto and kill a patient’s cancer cells. Therapeutic Vaccines are Vaccines to treat cancer take a variety of forms: Some are made from cancer cells, others from parts of cancer cells, and still others are from specially conditioned immune system cells. One type of cell-based vaccine involves removing certain immune system cells from a patient’s blood and converting them into dendritic cells, whose job is to display cancer- or infection-related proteins on their surface. The dendritic cells are combined with pieces of tumor cells and, often, other stimulatory proteins and infused back into the patient. The cancer-related proteins on the dendritic cells’ surface, called antigens, spur the patient’s immune system to go on the offensive against cancer cells. Live Vaccine (Attenuated)
Cancer ‘vaccine’ eliminates tumors in mice by Activating T cells in Tumors. Injecting microgram amounts of two immune-stimulating agents directly into solid tumors (A microgram is one-millionth of a gram). One, a short stretch of DNA called a CpG oligonucleotide, works with other nearby immune cells to amplify the expression of an activating receptor called OX40 on the surface of the T cells. The other, an antibody that binds to OX40, activates the T cells to lead the charge against the cancer cells. Because the two agents are injected directly into the tumor, only T cells that have infiltrated it are activated. In effect, these T cells are “prescreened” by the body to recognize only cancer-specific proteins.
Immune therapy takes a 'BiTE' out of brain cancer. The treatment, known as chimeric antigen receptor T-cell (CAR T) therapy, involves collecting and genetically modifying a patient's immune-fighting T cells to recognize specific targets (antigens) on the surface of tumors, and then returning them to the patient. Two CAR T cell products have been approved by the FDA for treatment of non-Hodgkin lymphoma and acute lymphoblastic leukemia, respectively cancers of the lymphatic system and blood.
Researchers Develop Synthetic T Cells, mimics form and function of human version.
Polymorphonuclear myeloid-derived suppressor cell (PMN-MDSC) express high levels of a surface protein known as FAS-ligand, which induces T cell suicide when it binds its receptor on T cells. The researchers show that depleting PMN-MDSCs from the tumors or blocking FAS-ligand binding to its receptor restored the ability of the T cells to kill induced tumors.
Lymphatic System is part of the circulatory system and a vital part of the immune system, comprising a network of lymphatic vessels that carry a clear fluid called lymph directionally towards the heart. (lympha from Latin meaning "water").
CAR T-Cell Gene Therapy Kymriah (tisagenlecleucel) is a living drug that involves using genetically modified T-cells or immune cells from patients to attack their cancer. Kymriah is a genetically-modified autologous T-cell immunotherapy. The patient’s T-cells are collected and sent to a manufacturing center where they are genetically modified to include a new gene that contains a specific protein (a chimeric antigen receptor or CAR) that directs the T-cells to target and kill leukemia cells that have a specific antigen (CD19) on the surface. Once the cells are modified, they are infused back into the patient to kill the cancer cells. Acute Lymphoblastic Leukemia is a cancer of the bone marrow and blood, in which the body makes abnormal lymphocytes, which is a type of white blood cell.
Chimeric Antigen Receptor T Cells are T cells that have been genetically engineered to produce an artificial T-cell receptor for use in immunotherapy. (also known as CAR T cells).
Researchers have cracked a code in T-cells that could make autoimmune diseases and organ transplant rejection a thing of the past. The scientists have identified a critical switch that controls T-cell function and dysfunction and have discovered a pathway to target it. Their study reveals targeting the IRF4 molecule in T-cells is the key to potentially curing most autoimmune diseases and eliminating Organ Transplant Rejection.
Mucosal Associated Invariant T cell makes up a subset of T cells in the immune system that display innate, effector-like qualities. In humans, MAIT cells are found in the blood, liver, lungs, and mucosa, defending against microbial activity and infection. The MHC class I-like protein, MR1, is responsible for presenting bacterially-produced vitamin B metabolites to MAIT cells. After the presentation of foreign antigen by MR1, MAIT cells secrete pro-inflammatory cytokines and are capable of lysing bacterially-infected cells. MAIT cells can also be activated through MR1-independent signaling. In addition to possessing innate-like functions, this T cell subset supports the adaptive immune response and has a memory-like phenotype. Furthermore, MAIT cells are thought to play a role in autoimmune diseases, such as multiple sclerosis, arthritis and inflammatory bowel disease, although definitive evidence is yet to be published.
Immunology is a branch of biology that covers the study of immune systems in all organisms.
Brain Immune System Link - Blood Brain Barrier
Virus-mimicking drug helps immune system target cunning cancer cells. Researchers found that a drug that activates the body's natural defenses by behaving like a virus may also make certain stealthy melanoma tumors visible to the immune system, allowing them to be better targeted by immunotherapy. Interferon's are proteins in cells that respond to viral infection by impeding the virus's ability to replicate and alerting the immune system to marshal its forces. Activating interferon signaling in tumors helps slow down tumor division and can lead to the release of molecules that recruit more immune cells to the tumor.
Interferon are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.
Fight cancer using someone else's immune cells
Toll-Like Receptor are a class of proteins that play a key role in the innate immune system. They are single, membrane-spanning, non-catalytic receptors usually expressed on sentinel cells such as macrophages and dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13, though the latter three are not found in humans.
Immunity - Autoimmunity
Immunity is the balanced state of having adequate biological defenses to fight infection, disease, or other unwanted biological invasion, while having adequate tolerance to avoid allergy, and autoimmune diseases. Above the Law.
Allergy are a number of conditions caused by hypersensitivity of the immune system to something in the environment that usually causes little or no problem in most people. These diseases include hay fever, food allergies, atopic dermatitis, allergic asthma, and anaphylaxis. Symptoms may include red eyes, an itchy rash, sneezing, a runny nose, shortness of breath, or swelling. Food intolerances and food poisoning are separate conditions.
Alloimmunity is an immune response to nonself antigens from members of the same species, which are called alloantigens or isoantigens. Two major types of alloantigens are blood group antigens and histocompatibility antigens. In alloimmunity, the body creates antibodies against the alloantigens, attacking transfused blood, allotransplanted tissue, and even the fetus in some cases. Alloimmune (isoimmune) response results in graft rejection, which is manifested as deterioration or complete loss of graft function. In contrast, autoimmunity is an immune response to the self's own antigens.
Autoimmunity is the system of immune responses of an organism against its own healthy cells and tissues. Any disease that results from such an aberrant immune response is termed an Autoimmune Disease.
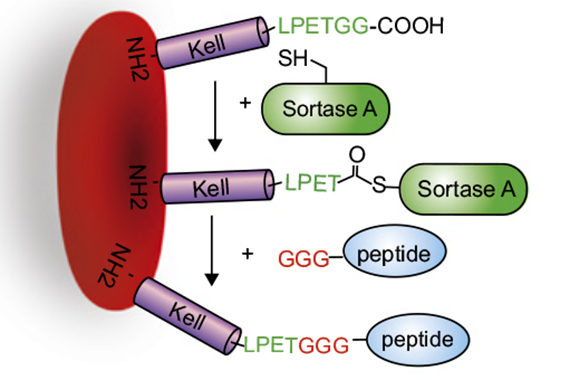 Autoimmune Disease is a condition arising from an abnormal
immune response to a normal body part. There are at least 80 types of
autoimmune diseases. Nearly any body part can be involved. Common symptoms
include low grade fever and feeling tired. Often symptoms come and go.
Autoimmune Disease is a condition arising from an abnormal
immune response to a normal body part. There are at least 80 types of
autoimmune diseases. Nearly any body part can be involved. Common symptoms
include low grade fever and feeling tired. Often symptoms come and go.Cargo-carrying Red Blood Cells alleviate Autoimmune Diseases in Mice.
Antibodies to Brain Proteins may Trigger Psychosis. Antibodies defend the body against bacterial, viral, and other invaders. But sometimes the body makes antibodies that attack healthy cells. In these cases, autoimmune disorders develop. Immune abnormalities in patients with psychosis have been recognized for over a century, but it has been only relatively recently that scientists have identified specific immune mechanisms that seem to directly produce symptoms of psychosis, including hallucinations and delusions. Body Burdened from Toxins.
Low Serotonin Levels have been linked to depression. Serotonin is an important chemical and neurotransmitter in the human body. It is believed to help regulate mood and social behavior, appetite and digestion, sleep, memory, and sexual desire and function. Gut Microbes.
Serotonin and Brain Development. The role of the serotonergic system in the neuroplastic events that create, repair, and degenerate the brain has been explored. Synaptic plasticity occurs throughout life and is critical during brain development. Evidence from biochemical, pharmacological, and clinical studies demonstrates the huge importance of an intact serotonergic system for normal central nervous system (CNS)function. Serotonin acts as a growth factor during embryogenesis, and serotonin receptor activity forms a crucial part of the cascade of events leading to changes in brain structure. The serotonergic system interacts with brain-derived neurotrophic factor (BDNF), S100beta, and other chemical messengers, in addition to ts cross talk with the GABAergic, glutamatergic, and dopaminergic neurotransmitter systems. Disruption of these processes may contribute to CNS disorders that have been associated with impaired development. Furthermore, many psychiatric drugs alter serotonergic activity and have been shown to create changes in brain structure with long-term treatment. However, the mechanisms for their therapeutic efficacy are still unclear. Treatments for psychiatric illness are usually chronic and alleviate psychiatric symptoms, rather than cure these diseases. Therefore, greater exploration of the serotonin system during brain development and growth could lead to real progress in the discovery of treatments for mental disorders.
Brain-Reactive Antibodies and Disease. Autoimmune diseases currently affect 5–7% of the world's population; in most diseases there are circulating autoantibodies. Brain-reactive antibodies are present in approximately 2–3% of the general population but do not usually contribute to brain pathology. These antibodies penetrate brain tissue only early in development or under pathologic conditions. This restriction on their pathogenicity and the lack of correlation between serum titers and brain pathology have, no doubt, contributed to a delayed appreciation of the contribution of autoantibodies in diseases of the central nervous system. Nonetheless, it is increasingly clear that antibodies can cause damage in the brain and likely initiate or aggravate multiple neurologic conditions; brain-reactive antibodies contribute to symptomatology in autoimmune disease, infectious disease, and malignancy.
Baking Soda may help reduce the destructive inflammation of autoimmune diseases like rheumatoid arthritis, scientists say. They have some of the first evidence of how the cheap, over-the-counter antacid can encourage our spleen to promote instead an anti-inflammatory environment that could be therapeutic in the face of inflammatory disease, scientists report.
How Immune Cells Kill Bacteria with Acid. Crucial protein for acidification of macrophage phagosome discovered.
Experimental Therapy Restores Nerve Insulation Damaged by Autoimmune Diseases.
Severe Combined Immunodeficiency (SCID) National Institute of Allergy and Infectious Diseases.
Pemphigus Vulgaris is a rare chronic blistering skin disease. It is classified as a type II hypersensitivity reaction, with the formation of antibodies against desmosomes, components of the skin that function to keep certain layers of skin bound to each other. As desmosomes are attacked, the layers of skin separate and the clinical picture resembles a blister. Over time the condition inevitably progresses without treatment: lesions increase in size and distribution throughout the body, behaving physiologically like a severe burn. Before the advent of modern treatments, mortality for the disease was close to 90%. Today, the mortality rate with treatment is between 5-15%.
Antigen is a molecule capable of inducing an immune response on the part of the host organism, though sometimes antigens can be part of the host itself. In other words, an antigen is any substance that causes an immune system to produce antibodies against it. Each antibody is specifically produced by the immune system to match an antigen after cells in the immune system come into contact with it; this allows a precise identification of the antigen and the initiation of a tailored response. The antibody is said to "match" the antigen in the sense that it can bind to it thanks to adaptations performed to a region of the antibody; because of this, many different antibodies can be produced, with specificity to bind many different antigens while sharing the same basic structure. In most cases, an antibody can only bind one specific antigen; in some instances, however, antibodies may bind more than one antigen. Self-Antigen is any molecule or chemical group of an organism which acts as an antigen in inducing antibody formation in another organism but to which the healthy immune system of the parent organism is tolerant. Blood Types.
Immunosenescence refers to the gradual deterioration of the immune system brought on by natural age advancement. It involves both the host's capacity to respond to infections and the development of long-term immune memory, especially by vaccination.
Immunodeficiency is a state in which the immune system's ability to fight infectious disease and cancer is compromised or entirely absent.
HIV - Aids
AIDS: Genetically-intact HIV hides in specific subsets of CD4+ T-cells. (effector memory T-cells). Specific immune memory T-cells where infectious HIV 'hides' in the human body to evade detection by the immune system. 36.9 million people were living with HIV in 2017. 940,000 people died of AIDS-related illnesses in 2017. 35.4 million people have died from AIDS-related illnesses since the epidemic was identified in 1981.
Aids or Human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS), is a spectrum of conditions caused by infection with the human immunodeficiency virus (HIV). Following initial infection, a person may not notice any symptoms or may experience a brief period of influenza-like illness. Typically, this is followed by a prolonged period with no symptoms. As the infection progresses, it interferes more with the immune system, increasing the risk of common infections like tuberculosis, as well as other opportunistic infections, and tumors that rarely affect people who have working immune systems. These late symptoms of infection are referred to as acquired immunodeficiency syndrome (AIDS). This stage is often also associated with weight loss. Aids Awareness.
Management of HIV-AIDS normally includes the use of multiple antiretroviral drugs in an attempt to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death.
Pre-Exposure Prophylaxis. Daily PrEP reduces the risk of getting HIV from sex by more than 90%.
New approach for AIDS: Lock HIV in reservoir cells to die through apoptosis. - Safe Sex.
The Dangerous Evolution of HIV: Edsel Salvaña 2017 (video and text)
Gene Therapy using CAR T-cells could provide long-term protection against HIV. Through gene therapy, researchers engineered blood-forming stem cells (hematopoietic stem/progenitor cells, or HSPCs) to carry chimeric antigen receptor (CAR) genes to make cells that can detect and destroy HIV-infected cells. These engineered cells not only destroyed the infected cells, they persisted for more than two years, suggesting the potential to create long-term immunity from the virus that causes AIDS. Because HIV uses CD4 to infect cells, the researchers used a CAR molecule that hijacks the essential interaction between HIV and the cell surface molecule CD4 to make stem cell-derived T-cells target infected cells. When the CD4 on the CAR molecule binds to HIV, other regions of the CAR molecule signal the cell to become activated and kill the HIV infected cell. The researchers found that, in test animals, modification of the blood-forming stem cells resulted in more than two years of stable production of CAR-expressing cells without any adverse effects. In addition, these cells were widely distributed throughout the lymphoid tissues and gastrointestinal tract, which are major anatomic sites for HIV replication and persistence in infected people. Most important, engineered CAR T-cells showed efficacy in attacking and killing HIV-infected cells.
HIV eliminated from the genomes of living animals. Researchers have for the first time eliminated replication-competent HIV-1 DNA -- the virus responsible for AIDS -- from the genomes of living animals. The study marks a critical step toward the development of a possible cure for human HIV infection.
Synthetic immunology: modulating the human immune system In synthetic immunology, biological devices are engineered to rationally modulate immune responses. Molecules derived from the immune system are modified to capture cytokines or cells Autologous immune cells are designed to cure immunodeficiencies or eradicate tumors.
Pattern Recognition Receptor are a primitive part of the immune system. They are proteins expressed by cells of the innate immune system to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with cell components that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity.
Cancer Immunology is a branch of immunology that studies interactions between the immune system and cancer cells (also called tumors or malignancies). It is a field of research that aims to discover cancer immunotherapies to treat and retard progression of the disease. The immune response, including the recognition of cancer-specific antigens, forms the basis of targeted therapy (such as vaccines and antibody therapies) and tumor marker-based diagnostic tests.
Cholesterol Byproduct Hijacks Immune Cells, lets Breast Cancer Spread
27-Hydroxycholesterol (wiki)
How to Help our Immune Cells to Recognize Cancer Cells.
What the Sugar Coating on your Cells is trying to tell you (video and interactive text)
Sialic Acid is a generic term for the N- or O-substituted derivatives of neuraminic acid, a monosaccharide with a nine-carbon backbone. It is also the name for the most common member of this group, N-acetylneuraminic acid (Neu5Ac or NANA). Sialic acids are found widely distributed in animal tissues and to a lesser extent in other organisms, ranging from plants and fungi to yeasts and bacteria, mostly in glycoproteins and gangliosides (they occur at the end of sugar chains connected to the surfaces of cells and soluble proteins). (Blood Type) In humans the brain has the highest sialic acid concentration, where these acids play an important role in neural transmission and ganglioside structure in synaptogenesis. In general, the amino group bears either an acetyl or a glycolyl group, but other modifications have been described. These modifications along with linkages have shown to be tissue specific and developmentally regulated expressions, so some of them are only found on certain types of glycoconjugates in specific cells. The hydroxyl substituents may vary considerably; acetyl, lactyl, methyl, sulfate, and phosphate groups have been found.
New cause of inflammation in people with HIV identified. After infection, HIV becomes a part of an infected person's DNA forever, and in most cases, infected cells are silent and do not replicate the virus. Occasionally, however, RNA is produced from this HIV DNA, which is a first step towards virus replication. Antiretroviral treatments help prevent HIV and AIDS-related complications, but they do not prevent the chronic inflammation that is common among people with HIV and is associated with mortality.
A single injection of a drug called cabotegravir given every two months has been shown to be more effective than a daily oral dose of Truvada. Truvada and cabotegravir are both antiretroviral drugs, medicines that have been incredibly effective in controlling HIV infections. A single injection of a drug called cabotegravir every two months was so successful in preventing HIV in a clinical trial among women in sub-Saharan Africa that the study was wrapped up ahead of schedule.
Discovery of Brain-like activity in immune system promises better disease treatments. Human immune cells contain particles that have neurotransmitters including dopamine, which plays a crucial role in immune responses. Neurons rely on synaptic interactions and neurotransmitters such as dopamine, which are small molecules transmitted across synapses to deliver signals from one cell to another that play a major role in reward-motivated behaviour. Like neurons, specialised T cells transfer dopamine to B cells that provides additional 'motivation' for B cells to produce the best antibodies they can to help to clear up an infection.
Cells
Plasma Cell are white blood cells that secrete large volumes of antibodies. They are transported by the blood plasma and the lymphatic system. Plasma cells originate in the bone marrow; B cells differentiate into plasma cells that produce antibody molecules closely modeled after the receptors of the precursor B cell. Once released into the blood and lymph, these antibody molecules bind to the target antigen (foreign substance) and initiate its neutralization or destruction.
B Cell are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system by secreting antibodies. Additionally, B cells present antigen (they are also classified as professional antigen-presenting cells (APCs)) and secrete cytokines. Bad antibodies in the immune system are usually 'silenced' in B cells because they can harm the body by attacking itself, can provide crucial protection against invading microbes.
Memory B Cell are a B cell sub-type that are formed within germinal centers following primary infection and are important in generating an accelerated and more robust antibody-mediated immune response in the case of re-infection (also known as a secondary immune response).
T Cell is a type of lymphocyte and a subtype of white blood cell that plays a central role in cell-mediated immunity, which is an immune response that does not involve antibodies. Rather, cell-mediated immunity is the activation of phagocytes, antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen. T cells can be distinguished from other lymphocytes, such as B cells and natural killer cells, by the presence of a T-cell receptor on the cell surface. They are called T cells because they mature in the thymus from thymocytes (although some also mature in the tonsils). The several subsets of T cells each have a distinct function. The majority of human T cells rearrange their alpha and beta chains on the cell receptor and are termed alpha beta T cells (αβ T cells) and are part of the adaptive immune system. Specialized gamma delta T cells, (a small minority of T cells in the human body, more frequent in ruminants), have invariant T cell receptors with limited diversity, that can effectively present antigens to other T cells and are considered to be part of the innate immune system.
Memory T Cell are the cells in the body that 'remember' previous infections and how to defeat them. These are the cells that provide life-long immunity to infections such as measles or chicken pox. Memory T Cells are a subset of infection- as well as potentially cancer-fighting T cells (also known as a T lymphocyte) that have previously encountered and responded to their cognate antigen; thus, the term antigen-experienced T cell is often applied. Such T cells can recognize foreign invaders, such as bacteria or viruses, as well as cancer cells. Memory T cells have become "experienced" by having encountered antigen during a prior infection, encounter with cancer, or previous vaccination. At a second encounter with the invader, memory T cells can reproduce to mount a faster and stronger immune response than the first time the immune system responded to the invader. This behavior is utilized in T lymphocyte proliferation assays, which can reveal exposure to specific antigens.
G.M. T Cell Therapy
Cytotoxic T Cell is a T lymphocyte or a type of white blood cell that kills cancer cells, cells that are infected, particularly with viruses, or cells that are damaged in other ways.
T Helper Cell are a type of T cell that play an important role in the immune system, particularly in the adaptive immune system. They help the activity of other immune cells by releasing T cell cytokines. These cells help suppress or regulate immune responses. They are essential in B cell antibody class switching, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages.
Sunlight Energizes Infection Fighting T Cells
Scientists find molecular key to body making healthy T cells. Researchers seek new therapies to stop immune diseases. For T cells to activate and mature, the scientists say AP-1 must help open up chromatin -- the twisting structure of DNA that winds around and condenses itself in the cell nucleus to control the cell. This stimulates a cascade of genetic and molecular programs that cooperate to form the cells. In experiments where the scientists inhibited AP-1 in early CD4 T cells, the chromatin didn't open as it's supposed to and the T cells didn't form or function correctly. What makes the finding significant is the AP-1 transcription factor accumulates and influences molecular processes at sites called risk loci. Risk loci are locations on the chromatin that are prone to genetic mutation and linked to multiple immune diseases, such as inflammatory bowel disease, allergies or the neurodegenerative condition multiple sclerosis. The research involves a multidisciplinary collaboration of different teams, including scientists in the Center for Autoimmune Genomics and Etiology and the division of Biomedical Informatics and Genetics. Combining different biological and computational methods of analysis, the research team was able to profile the accessibility of chromatin to molecular remodeling during the early stages of CD4 T cell activation. They used technologies called ChIP-seq (chromatin immunoprecipitation) and ATAC-Seq (Assay for Transposase Accessible Chromatin) that analyze all the different protein interactions with DNA and chromatin state. This allowed the researchers to identify the specific binding sites on chromatin of DNA-associated proteins. Combining the experimental and computational analysis revealed AP-1 binding to DNA during the early stages of CD4 T cell activation. The researchers show when AP-1 does bind to certain chromatin locations, it does so in conjunction with its molecular partner, NFAT1 (nuclear factor of activated T cells). The researchers said earlier studies have focused on genetic disruption of individual members AP-1 family proteins, of which there are eighteen. In the current study however, the researchers said they broadly blocked the entire AP-1 family of proteins in human naïve T cells. When study authors inhibited AP-1, they said it prevented chromatin changes that normally occur in the cell nucleus and the T failed to activate. Now Barski and his colleagues are working to find out if the absence of AP-1 contributes to people acquiring an immune disease when AP-1 is unable to bind with mutated loci. Barski says the scientists want to use new information from their future experiments to look for new, groundbreaking therapies that could change the outcome for patients.
Thymus is a specialized primary lymphoid organ of the immune system. Within the thymus, T cells or T lymphocytes mature. T cells are critical to the adaptive immune system, where the body adapts specifically to foreign invaders. The thymus is composed of two identical lobes and is located anatomically in the anterior superior mediastinum, in front of the heart and behind the sternum.
Ipilimumab
Natural Killer Cell are a type of cytotoxic lymphocyte critical to the innate immune system. The role NK cells play is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK Cells provide rapid responses to viral-infected cells, acting at around 3 days after infection, and respond to tumor formation. Typically, immune cells detect major histocompatibility complex (MHC) presented on infected cell surfaces, triggering cytokine release, causing lysis or apoptosis. NK cells are unique, however, as they have the ability to recognize stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the initial notion that they do not require activation to kill cells that are missing "self" markers of MHC class 1. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells. NK cells (belonging to the group of innate lymphoid cells) are defined as large granular lymphocytes (LGL) and constitute the third kind of cells differentiated from the common lymphoid progenitor-generating B and T lymphocytes. NK cells are known to differentiate and mature in the bone marrow, lymph nodes, spleen, tonsils, and thymus, where they then enter into the circulation. NK cells differ from natural killer T cells (NKTs) phenotypically, by origin and by respective effector functions; often, NKT cell activity promotes NK cell activity by secreting interferon gamma. In contrast to NKT cells, NK cells do not express T-cell antigen receptors (TCR) or pan T marker CD3 or surface immunoglobulins (Ig) B cell receptors, but they usually express the surface markers CD16 (FcγRIII) and CD56 in humans, NK1.1 or NK1.2 in C57BL/6 mice. The NKp46 cell surface marker constitutes, at the moment, another NK cell marker of preference being expressed in both humans, several strains of mice (including BALB/c mice) and in three common monkey species. In addition to the knowledge that natural killer cells are effectors of innate immunity, recent research has uncovered information on both activating and inhibitory NK cell receptors which play important functional roles, including self tolerance and the sustaining of NK cell activity. NK cells also play a role in the adaptive immune response: numerous experiments have demonstrated their ability to readily adjust to the immediate environment and formulate antigen-specific immunological memory, fundamental for responding to secondary infections with the same antigen. The role of NK cells in both the innate and adaptive immune responses is becoming increasingly important in research using NK cell activity as a potential cancer therapy.
The Immune System's Super-Cell. NK cells, or natural killer cells, play an important role in the body's defences against cancer and various infections. Now scientists have mapped how the different steps of the maturation process of these supercells from blood producing stem cells in the bone marrow are regulated: knowledge which is crucial for the development of new immunotherapies against cancer.
Natural Killer T Cell are a heterogeneous group of T cells that share properties of both T cells and natural killer cells. Many of these cells recognize the non-polymorphic CD1d molecule, an antigen-presenting molecule that binds self and foreign lipids and glycolipids. They constitute only approximately 0.1% of all peripheral blood T cells. Natural killer T cells should not be confused with natural killer cells.
Stem Cells - Natural Defenses
Mast Cell is a resident cell of connective tissue that contains many granules rich in histamine and heparin. Specifically, it is a type of granulocyte derived from the myeloid stem cell that is a part of the immune and neuroimmune systems . Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in wound healing, angiogenesis, immune tolerance, defense against pathogens, and blood–brain barrier function. The mast cell is very similar in both appearance and function to the basophil, another type of white blood cell. Although mast cells were once thought to be tissue resident basophils, it has been shown that the two cells develop from different hematopoietic lineages and thus cannot be the same cells.
Dendritic Cell are antigen-presenting cells (also known as accessory cells) of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and the adaptive immune systems. Dendritic cells are present in those tissues that are in contact with the external environment, such as the skin (where there is a specialized dendritic cell type called the Langerhans cell) and the inner lining of the nose, lungs, stomach and intestines. They can also be found in an immature state in the blood. Once activated, they migrate to the lymph nodes where they interact with T cells and B cells to initiate and shape the adaptive immune response. At certain development stages they grow branched projections, the dendrites that give the cell its name (δένδρον or déndron being Greek for 'tree'). While similar in appearance, these are structures distinct from the dendrites of neurons. Immature dendritic cells are also called veiled cells, as they possess large cytoplasmic 'veils' rather than dendrites.
Psychoneuroimmunology is the study of the interaction between psychological processes and the nervous and immune systems of the human body. PNI takes an interdisciplinary approach, incorporating psychology, neuroscience, immunology, physiology, genetics, pharmacology, molecular biology, psychiatry, behavioral medicine, infectious diseases, endocrinology, and rheumatology.
Polysaccharide are polymeric carbohydrate molecules composed of long chains of monosaccharide units bound together by glycosidic linkages and on hydrolysis give the constituent monosaccharides or oligosaccharides. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch and glycogen, and structural polysaccharides such as cellulose and chitin.
Properdin is the only known positive regulator of complement activation that stabilizes the alternative pathway convertases. It is found in the blood serum of more complex animals.
Phagocyte are cells that protect the body by ingesting (phagocytosing) harmful foreign particles, bacteria, and dead or dying cells.
Lymphocyte is one of the subtypes of white blood cell in a vertebrate's immune system. Lymphocytes include natural killer cells (NK cells) (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic adaptive immunity), and B cells (for humoral, antibody-driven adaptive immunity). They are the main type of cell found in lymph, which prompted the name "lymphocyte".
Cytokine Storm is a potentially fatal immune reaction consisting of a positive feedback loop between cytokines and white blood cells, with highly elevated levels of various cytokines.
Hygiene - Vaccines
Cytokine are a broad and loose category of small proteins (~5–20 kDa) that are important in cell signaling. Their release has an effect on the behavior of cells around them. Cell Death.
Food - Nutrition - Beneficial Diets
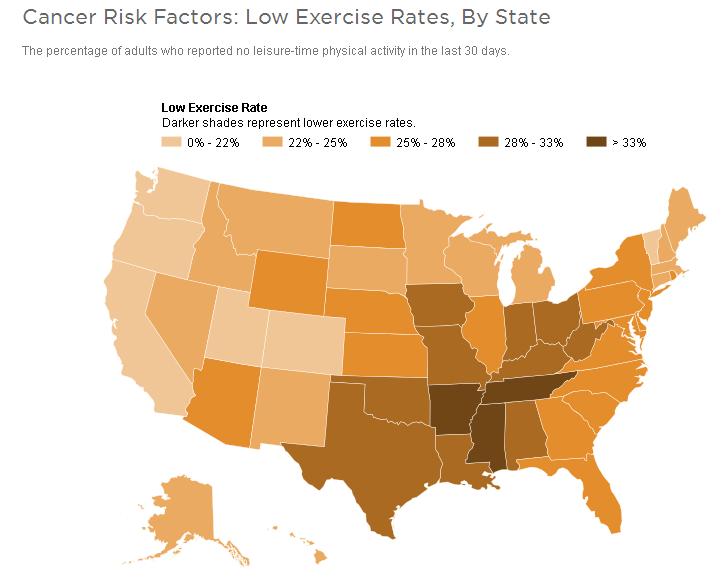 Avoiding
Foods with Processed Sugar
is a good idea because Cancer cells usually grow quickly, multiplying at a
fast rate, which takes a lot of energy. This means they need lots of
glucose. But all our healthy cells need glucose too, so eliminating foods
with natural sugar won’t stop cancer cells from dividing. Diets high in
sugar and refined carbohydrates can lead to overweight and obesity, which
indirectly increases cancer risk over time. When reading food labels, look
for sugar listed as the first ingredient and be aware of hidden
sugar names: fructose, lactose,
sucrose, maltose, glucose, dextrose. Natural sugars—molasses, agave
nectar, honey and maple syrup—contain beneficial antioxidants but those,
too, should be consumed in moderation.
Research suggests that glucose depletion therapies might work, as long as
the cancer cells produce
PKCz.
Avoiding
Foods with Processed Sugar
is a good idea because Cancer cells usually grow quickly, multiplying at a
fast rate, which takes a lot of energy. This means they need lots of
glucose. But all our healthy cells need glucose too, so eliminating foods
with natural sugar won’t stop cancer cells from dividing. Diets high in
sugar and refined carbohydrates can lead to overweight and obesity, which
indirectly increases cancer risk over time. When reading food labels, look
for sugar listed as the first ingredient and be aware of hidden
sugar names: fructose, lactose,
sucrose, maltose, glucose, dextrose. Natural sugars—molasses, agave
nectar, honey and maple syrup—contain beneficial antioxidants but those,
too, should be consumed in moderation.
Research suggests that glucose depletion therapies might work, as long as
the cancer cells produce
PKCz. Food Knowledge - Nutrition Knowledge
The Gerson Miracle (youtube) - Modified Gerson Approach
Dr. William Li (youtube) - Mina Bissell (video)
Burzynski Clinic
Personalized Nutrition - Vegetable Smoothies
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels. In precise usage this is distinct from vasculogenesis, which is the de novo formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise (especially in older texts). The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease. Angiogenesis is a normal and vital process in growth and development, as well as in wound healing and in the formation of granulation tissue. However, it is also a fundamental step in the transition of tumors from a benign state to a malignant one, leading to the use of angiogenesis inhibitors in the treatment of cancer. The essential role of angiogenesis in tumor growth was first proposed in 1971 by Judah Folkman, who described tumors as "hot and bloody," illustrating that, at least for many tumor types, flush perfusion and even hyperemia are characteristic. Angiogenesis Foundation.
Vitamins - Nutrition - Garlic - Probiotics - Microbiology -
Proteins Restricts Tumor Growth (PDF)
Breast Cancer Linked to Bacterial Imbalances - Gut Microbes
Cannabis - Medical Marijuana - Longevity Diets (benefits of eating healthier)
Bioactive Novel Compounds from Endangered Tropical Plant Species. Biologists have isolated 17 secondary metabolites, including three novel compounds from the valuable endangered tropical plant species Alangium longiflorum. A newly isolated compound, 8-hydroxytubulosine, showed growth inhibitory effects at submicromolar levels against several human tumor cell lines except for drug transporter-overexpressing cells. Compound 1 caused accumulation of sub-G1 cells with no effect on cell cycle progression, suggesting that this substance is an apoptosis inducer. Many antitumor drugs currently in clinical use, such as paclitaxel, vinca alkaloids (vinblastine and vincristine), podophyllotoxin analogues (etoposide and teniposide), and topotecan (camptothecin analog) are based on natural plants. According to a report, 83% of new chemical entities identified as anticancer agents from 1981 to 2014 were derived from natural products.
Trophoblast are cells forming the outer layer of a blastocyst, which provide nutrients to the embryo and develop into a large part of the placenta. They are formed during the first stage of pregnancy and are the first cells to differentiate from the fertilized egg. This layer of trophoblasts is also collectively referred to as "the trophoblast", or, after gastrulation, the trophectoderm, as it is then contiguous with the ectoderm of the embryo.
Mistletoe is the common name for most obligate hemiparasitic plants in the order Santalales. Mistletoes attach to and penetrate the branches of a tree or shrub by a structure called the haustorium, through which they absorb water and nutrients from the host plant.
Amygdalin is a poisonous cyanogenic glycoside found in many plants, but most notably in the seeds (kernels) of apricot, bitter almonds, apple, peach, and plum. B-17.
Ketosis is a metabolic state in which some of the body's energy supply comes from ketone bodies in the blood, in contrast to a state of glycolysis in which blood glucose provides most of the energy. Fasting.
Ketone Bodies are three water-soluble molecules (acetoacetate, beta-hydroxybutyrate, and their spontaneous breakdown product, acetone) containing the ketone group that are produced by the liver from fatty acids during periods of low food intake (fasting), carbohydrate restrictive diets, starvation, prolonged intense exercise, alcoholism or in untreated (or inadequately treated) type 1 diabetes mellitus. These ketone bodies are readily picked up by the extra-hepatic tissues (tissues outside the liver) and converted into acetyl-CoA which then enters the citric acid cycle and is oxidized in the mitochondria for energy. In the brain, ketone bodies are also used to make acetyl-CoA into long-chain fatty acids. Ketone bodies are produced by the liver under the circumstances listed above (i.e. fasting, starving, low carbohydrate diets, prolonged exercise and untreated type 1 diabetes mellitus) as a result of intense gluconeogenesis, which is the production of glucose from non-carbohydrate sources (not including fatty acids). They are therefore always released into the blood by the liver together with newly produced glucose after the liver glycogen stores have been depleted (these glycogen stores are depleted within the first 24 hours of fasting). When two acetyl-CoA molecules lose their -CoAs, (or Co-enzyme A groups) they can form a (covalent) dimer called acetoacetate. Beta-hydroxybutyrate is a reduced form of acetoacetate, in which the ketone group is converted into an alcohol (or hydroxyl) group. Both are 4-carbon molecules, that can readily be converted back into acetyl-CoA by most tissues of the body, with the notable exception of the liver. Acetone is the decarboxylated form of acetoacetate which cannot be converted back into acetyl-CoA except via detoxification in the liver where it is converted into lactic acid, which can, in turn, be oxidized into pyruvic acid, and only then into acetyl-CoA. Ketone bodies have a characteristic smell, which can easily be detected in the breath of persons in ketosis and ketoacidosis. It is often described as fruity or like nail polish remover (which usually contains acetone or ethyl acetate). Apart from the three endogenous ketone bodies, acetone, acetoacetic acid, and beta-hydroxybutyric acid, other ketone bodies like beta-ketopentanoate and beta-hydroxypentanoate may be created as a result of the metabolism of synthetic triglycerides, such as triheptanoin. In the brain, ketone bodies are also used to make acetyl-CoA into long-chain fatty acids. These ketone bodies are readily picked up by the extra-hepatic tissues, and converted into acetyl-CoA which then enters the citric acid cycle and is oxidized in the mitochondria for energy.
Ketogenic Diet is a high-fat, adequate-protein, low-carbohydrate diet that in medicine is used primarily to treat difficult-to-control (refractory) epilepsy in children. The diet forces the body to burn fats rather than carbohydrates. Normally, the carbohydrates contained in food are converted into glucose, which is then transported around the body and is particularly important in fueling brain-function. However, if there is very little carbohydrate in the diet, the liver converts fat into fatty acids and ketone bodies. The ketone bodies pass into the brain and replace glucose as an energy source. An elevated level of ketone bodies in the blood, a state known as ketosis, leads to a reduction in the frequency of epileptic seizures. Hope for Health.
Starving Cancer Cells using a key nutrient Amino Acids, slows Tumor Growth
Serine is an amino acid that is used in the biosynthesis of proteins.
TP53 is any isoform of a protein encoded by homologous genes in various organisms, such as TP53 (humans) and Trp53 (mice).
Metabolic Therapy believe that each person has a unique Metabolism, and that the proportion of macromolecules (proteins, carbohydrates and fats) which are optimal for one person may not be for a second, and could even be detrimental to them.
Links between Metabolism and Cancer
Lipid Metabolism is the synthesis and degradation of lipids in cells. Lipid metabolism is the break down or storage of fats for energy; these fats are obtained from consuming food and absorbing them or they are synthesized by an animal's liver. Aberrant Lipid Metabolism in the Forebrain Niche Suppresses Adult Neural Stem Cell Proliferation in an Animal Model of Alzheimer’s Disease.
Growing Evidence Points to a Specific Diet That Can Starve Cancer Cells of Their Prime Fuels. The Metabolic Approach to Cancer: Integrating Deep Nutrition, the Ketogenic Diet, and Nontoxic Bio-Individualized Therapies.
Marine phospholipids--a promising new dietary approach to tumor-associated weight loss.
Cancer: The Forbidden Cures (youtube)
Extracellular Matrix is a collection of extracellular molecules secreted by cells that provides structural and biochemical support to the surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
Essiac is an herbal tea promoted as an alternative treatment for cancer and other illnesses. There is no evidence it is beneficial to health, and it may be harmful. Essiac Info.
Organic Health - Laetrile - Laetrile Research Film
Podophyllum or Mayapple, all the parts of the plant are poisonous, including the green fruit, but once the fruit has turned yellow, it can be safely eaten with the seeds removed.
New Health Benefits discovered in Berry Pigment. Naturally occurring pigments in berries, also known as anthocyanins, increase the function of the sirtuin 6 enzyme in cancer cells, a new study shows. The regulation of this enzyme could open up new avenues for cancer treatment.
Anthocyanin are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, or blue. Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.
Sirtuin 6 is a stress responsive protein deacetylase and mono-ADP ribosyltransferase enzyme encoded by the SIRT6 gene. SIRT6 functions in multiple molecular pathways related to aging, including DNA repair, telomere maintenance, glycolysis and inflammation.
Queensland Berry cancer-fighting berry on tree that only grows in Far North Queensland. EBC-46.
Endothelium is a type of epithelium that lines the interior surface of blood vessels and lymphatic vessels, forming an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. It is a thin layer of simple squamous cells called endothelial cells. Endothelial cells in direct contact with blood are called vascular endothelial cells, whereas those in direct contact with lymph are known as lymphatic endothelial cells. Vascular endothelial cells line the entire circulatory system, from the heart to the smallest capillaries. These cells have unique functions in vascular biology. These functions include fluid filtration, such as in the glomerulus of the kidney, blood vessel tone, hemostasis, neutrophil recruitment, and hormone trafficking. Endothelium of the interior surfaces of the heart chambers is called endocardium.
Psilocybin Mushrooms are mushrooms that contain the psychedelic compounds psilocybin and psilocin. Common colloquial terms include magic mushrooms and shrooms. They are used mainly as an entheogen and recreational drug whose effects can include euphoria, altered thinking processes, closed and open-eye visuals, synesthesia, an altered sense of time and spiritual experiences. Biological genera containing psilocybin mushrooms include Copelandia, Galerina, Gymnopilus, Inocybe, Mycena, Panaeolus, Pholiotina, Pluteus, and Psilocybe. Over 100 species are classified in the genus Psilocybe. Psilocybin mushrooms may have been used since prehistoric times. They are possibly depicted in Stone Age rock art in Europe and Africa, and have a history of use in pre-Columbian Mesoamerica. Many cultures have used these mushrooms in their religious rites and ceremonie. News.
Candida Fungus is a genus of yeasts and is the most common cause of fungal infections worldwide. Many species are harmless commensals or endosymbionts of hosts including humans; however, when mucosal barriers are disrupted or the immune system is compromised they can invade and cause disease. Candida albicans is the most commonly isolated species, and can cause infections (candidiasis or thrush) in humans and other animals. In winemaking, some species of Candida can potentially spoil wines.
Keyhole Limpet Hemocyanin is a large, multisubunit, oxygen-carrying, metalloprotein that is found in the hemolymph of the giant keyhole limpet, Megathura crenulata, a species of keyhole limpet that lives off the coast of California, from Monterey Bay to Isla Asuncion off Baja California.
Antineoplaston Therapy
Caris Life Sciences
Cancer Tutor
Peptides are biologically occurring short chains of amino acid monomers linked by peptide (amide) bonds.
Reactive Oxygen species are chemically reactive chemical species containing oxygen. Examples include peroxides, superoxide, hydroxyl radical, and singlet oxygen.
Allergies - Wellness - Keytruda
Chimera is a single organism composed of cells from different zygotes. This can result in male and female organs, two blood types, or subtle variations in form. Animal chimeras are produced by the merger of multiple fertilized eggs.
Therapies for Cancer
Cell Therapy is therapy in which cellular material is injected into a patient; this generally means intact, living cells. For example, T cells capable of fighting cancer cells via cell-mediated immunity may be injected in the course of immunotherapy.
Gene Therapy is the therapeutic delivery of nucleic acid polymers into a patient's cells as a drug to treat disease.
Pembrolizumab is a humanized antibody used in cancer immunotherapy. It destroys a protective mechanism on cancer cells, and allows the immune system to destroy those cancer cells. It targets the programmed cell death 1 (PD-1) receptor. The drug was initially used in treating metastatic melanoma.
Personalized Cell Therapies
Juno Therapeutics engineers immune cells that can detect proteins and target cancer cells specifically.
City of Hope
Sloan Kettering
Cancer Research
Molecular Cancer Therapeutics
Molecular Cancer
International Agency for Research on Cancer
Cancer Genes Turned Off
Intravenous Immunoglobulin is the use of a mixture of antibodies (immunoglobulins) to treat a number of health conditions.
Staphylococcus Lugdunensis is a coagulase-negative member of the genus Staphylococcus, consisting of Gram-positive bacteria with spherical cells that appear in clusters.
Engineered Liposomes Sequester Bacterial Exotoxins
Liposome is a spherical vesicle having at least one lipid bilayer. The liposome can be used as a vehicle for administration of nutrients and pharmaceutical drugs. Liposomes can be prepared by disrupting biological membranes (such as by sonication).
Exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host-pathogen interface.
Sipuleucel-T is a cell-based cancer immunotherapy for prostate cancer (CaP). It is a personalized treatment that works by programming each patient's immune system to seek out cancer and attack it as if it were foreign.
Platelet-Rich Plasma is blood plasma that has been enriched with platelets. As a concentrated source of autologous platelets, PRP contains several different growth factors and other cytokines that can stimulate healing of soft tissue. Platelet-rich plasma therapy is an old therapy and used extensively in specialities of dermatology, orthopedics and dentistry. Platelet rich plasma therapy utilizes growth factors present in alpha granules of platelets in an autologous manner. Main indications in dermatology for PRP are androgenetic alopecia, wound healing, face rejuvenation etc. For preparation of PRP, various protocols are used and no standard protocol exists but main principles essentially involve concentrating platlets in a concentration of 3–5 times the physiological value and then injecting this concentrated plasma in the tissue where healing or effect is desired. As of 2016, no large-scale randomized controlled trials have confirmed the efficacy of PRP as a treatment for musculoskeletal or nerve injuries, the accelerated healing of bone grafts, or the reduction of androgenic hair loss.
Platelet-Rich Plasma (PRP) Therapy
Transfusion Cellular Therapies
Offering abiraterone (Zytiga) up-front with standard hormone treatment extends survival in men whose prostate cancer has spread
Combined immunotherapy could help control melanoma that has spread to the brain
Stimulating Defense Mechanisms
Naturally occurring molecule enhances defense mechanisms in neurodegenerative diseases
N-Acetylglucosamine is a monosaccharide and a derivative of glucose. It is an amide between glucosamine and acetic acid.
Hexosamine Pathway Metabolites Enhance Protein Quality Control and Prolong Life.
Telomere is a region of repetitive nucleotide sequences at each end of a chromosome, which protects the end of the chromosome from deterioration or from fusion with neighboring chromosomes.
Rare byproduct of marine bacteria lomaiviticin A kills cancer cells by snipping their DNA
Alternative Cancer Treatments are alternative or complementary treatments for cancer that have not been approved by the government agencies responsible for the regulation of therapeutic goods.
Tumor Treating Fields is a type of electromagnetic field therapy using low-intensity electrical fields to treat cancer.
Alternating Electric Field Therapy is a type of electromagnetic field therapy using low-intensity electrical fields to treat cancer. A TTF-generating device manufactured by the Israeli company Novocure is approved in the United States and Europe for the treatment of newly diagnosed and recurrent glioblastoma multiforme (GBM), and is undergoing clinical trials for several other tumor types. Despite earning regulatory approval, the efficacy of this technology remains controversial among medical experts. Sometimes called tumor treating fields TTF or TTFields.
Electromagnetic Therapy refers to therapy involving the use of magnets or electromagnets. Types include: Bioelectromagnetics, the study of how electromagnetic fields interact with and influence biological processes. Electrotherapy, the use of electrical or electromagnetic energy in medicine; Electromagnetic therapy (alternative medicine), the use of electromagnetic radiation to treat disease. Evidence of efficacy is lacking. Pulsed Electromagnetic Field Therapy, or PEMF, the use of weak electromagnetic fields to initiate osteogenesis. Alternating electric field therapy, also known as "Tumor Treating Fields", the use of electric fields as an anti-mitotic therapy for cancer patients.
Photothermal Therapy refers to efforts to use electromagnetic radiation (most often in infrared wavelengths) for the treatment of various medical conditions, including cancer. This approach is an extension of photodynamic therapy, in which a photosensitizer is excited with specific band light. This activation brings the sensitizer to an excited state where it then releases vibrational energy (heat), which is what kills the targeted cells. Unlike photodynamic therapy, photothermal therapy does not require oxygen to interact with the target cells or tissues. Current studies also show that photothermal therapy is able to use longer wavelength light, which is less energetic and therefore less harmful to other cells and tissues.
Targeted treatment of cancer with Radiofrequency Electromagnetic Fields amplitude-modulated at Tumor-Specific Frequencies. There have been many attempts to treat cancer with low levels of electric and magnetic fields. We have developed noninvasive biofeedback examination devices and techniques and discovered that patients with the same tumor type exhibit biofeedback responses to the same, precise frequencies. Intrabuccal administration of 27.12 MHz radiofrequency (RF) electromagnetic fields (EMF), which are amplitude-modulated at tumor-specific frequencies, results in long-term objective responses in patients with cancer and is not associated with any significant adverse effects. Intrabuccal administration allows for therapeutic delivery of very low and safe levels of EMF throughout the body as exemplified by responses observed in the femur, liver, adrenal glands, and lungs. In vitro studies have demonstrated that tumor-specific frequencies identified in patients with various forms of cancer are capable of blocking the growth of tumor cells in a tissue- and tumor-specific fashion. Current experimental evidence suggests that tumor-specific modulation frequencies regulate the expression of genes involved in migration and invasion and disrupt the mitotic spindle. This novel targeted treatment approach is emerging as an appealing therapeutic option for patients with advanced cancer given its excellent tolerability. Dissection of the molecular mechanisms accounting for the anti-cancer effects of tumor-specific modulation frequencies is likely to lead to the discovery of novel pathways in cancer.
Electric Fields (youtube)
Fake Cancer Cures
Bogus Cancer Treatments (youtube)
Ernst T. Krebs was an American biochemist. He is known for promoting various substances as alternative cures for cancer.
Hoxsey Therapy is an alternative medical treatment promoted as a cure for cancer.
Ryke Geerd Hamer is a German former physician, a system of pseudo-medicine that purports to be able to cure cancer.
Cancer Misdiagnosis Guide
Witch Doctor is a type of healer who treated ailments believed to be caused by witchcraft, which is the practice of, and belief in, magical skills and abilities that are able to be exercised by individuals and certain social groups.
Placebos
Testing Treatments critical thinking about treatment claims.
Effects of the Informed Health Choices podcast on the ability of parents of primary school children in Uganda to assess claims about treatment effects: a randomized controlled trial.
Chemo
Chemotherapy is a category of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents) as part of a standardized chemotherapy regimen. Each year, about 650,000 cancer patients receive chemotherapy in an outpatient oncology clinic in the United States. Chemotherapy Types.
Chemotherapy Regimen is a regimen for chemotherapy, defining the drugs to be used, their dosage, the frequency and duration of treatments, and other considerations. In modern oncology, many regimens combine several chemotherapy drugs in combination chemotherapy. The majority of drugs used in cancer chemotherapy are cytostatic, many via cytotoxicity.
Because chemotherapy drugs travel through the body, they can also impact healthy cells, leading to a variety of side effects. Chemo is designed to kill fast-growing cancer cells, but this can sometimes lead to side effects involving the body’s other, healthy fast-growing cells. Blood forming cells in the bone marrow (anemia, increased risk of infection, bruising). Hair follicles (temporary hair loss) Cells in the mouth, digestive and reproductive tract (nausea, loss appetite, constipation, diarrhea) Some chemo drugs can damage cells in the heart, kidneys, bladder, lungs, and nervous system. Your doctor monitors you closely and may prescribe medicines to protect your body’s normal cells. There are also medicines to help relieve side effects.
Mitochondria are the 'canary in the coal mine' for cellular stress. How some cancers resist chemotherapy. Mitochondria, tiny structures present in most cells, are known for their energy-generating machinery. Now, researchers have discovered a new function of mitochondria: they set off molecular alarms when cells are exposed to stress or chemicals that can damage DNA, such as chemotherapy. The results could lead to new cancer treatments that prevent tumors from becoming resistant to chemotherapy.
Cytostasis is the inhibition of cell growth and multiplication.
Cell Division
Cytotoxicity is the quality of being toxic to cells.
MOPP Chemotherapy is a combination chemotherapy regimen used to treat Hodgkin's disease. The acronym is derived from the component drugs of the regimen:(M)ustargen (also known as mechlorethamine, chlormethine, mustine, nitrogen mustard, or MSD). (O)ncovin (also known as Vincristine or VCR). (P)rocarbazine (also known as Matulane or Natulan). (P)rednisone (also known as Deltasone or Orasone). The treatment is usually administered in four week cycles, often for six cycles. MSD and VCR are administered intravenously, while procarbazine and prednisone are pills taken orally. A newer Hodgkin's lymphoma treatment is ABVD. C-MOPP involves switching the nitrogen mustard from mechlorethamine to cyclophosphamide. C-MOPP is thus very similar to COPP, using the same 4 agents and differing at most in dosages and timing.
Chemo Brain describes the cognitive impairment that can result from chemotherapy treatment. Approximately 20–30% of people who undergo chemotherapy experience some level of post-chemotherapy cognitive impairment. The phenomenon first came to light because of the large number of breast cancer survivors who complained of changes in memory, fluency, and other cognitive abilities that impeded their ability to function as they had pre-chemotherapy.
Fatigue
Over Saturation Warnings from Medications
Radiation Therapy is therapy using ionizing radiation, generally as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor (for example, early stages of breast cancer). Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. Radiation therapy is the use of high-energy particles or waves to destroy or damage cancer cells. Radiation is delivered using special equipment that sends high doses of radiation to the cancer cells or tumor. Radiation can also affect healthy cells, however, normal cells can repair themselves, while cancer cells cannot.
Hypoxia-Inducible Factors are transcription factors that respond to decreases in available oxygen in the cellular environment, or hypoxia.
Dimethyloxalylglycine - DMOG Treatment
Focused Ultrasound to Dissolve Tumors. (HIFU)
High Intensity Focused Ultrasound is an early stage medical technology that is in various stages of development worldwide to treat a range of disorders. The mechanism is similar to using a magnifying glass to focus sunlight. Focused ultrasound uses an acoustic lens to concentrate multiple intersecting beams of ultrasound on a target. Each individual beam passes through tissue with little effect but at the focal point where the beams converge, the energy can have useful thermal or mechanical effects. HIFU is typically performed with real-time imaging via ultrasound or MRI to enable treatment targeting and monitoring (including thermal tracking with MRI).
Chemoreceptor is a specialized cell which acts as a sensory receptor which transduces (responds to) a chemical substance and generates a biological signal. This signal may be in the form of an action potential if the chemoreceptor is a neuron (nerve cell), or in the form of a neurotransmitter that can activate a nearby nerve fiber if the chemosensor is a specialized sensory cell, such as a sensory cell in a taste bud or in an internal chemoreceptor such as the carotid body. In more general terms, a chemosensor detects chemicals in the internal or external environment and transmits that information to the nervous system.
Peripheral Chemoreceptors are so named because they are sensory extensions of the peripheral nervous system into blood vessels where they detect changes in chemical concentrations. As transducers of patterns of variability in the surrounding environment, carotid and aortic bodies count as ‘sensors’ in a similar way as taste buds and photoreceptors. However, because carotid and aortic bodies detect variation within the body’s internal organs, they are considered interoceptors. Taste buds, olfactory bulbs, photoreceptors, and other receptors associated with the five traditional sensory modalities, by contrast, are exteroceptors in that they respond to stimuli outside the body. The body also contains proprioceptors, which respond to the amount of stretch within the organ, usually muscle, that they occupy.
Pathwork Molecular Diagnostics - Nano-Medicine
Alternating Electric Field Therapy is a type of electromagnetic field therapy using low-intensity electrical fields to treat cancer.
Experimental Cancer Treatment are medical therapies intended or claimed to treat cancer (see also tumor) by improving on, supplementing or replacing conventional methods (surgery, chemotherapy, radiation, and immunotherapy).
Antibiotics - Allergies - Viruses
You need to do a lot of research when seeking out cancer treatments. And don't believe that Doctors are looking out for your best interest because sadly too many Doctors are corrupted by money.
The Cure is Worse than the Disease is when the medical treatment for an illness produces a worse net result than the illness does (threatens a non-negligible risk of doing so), especially via adverse effects. (figuratively) The solution or proposed solution to a problem produces a worse net result than the problem does (threatens a non-negligible risk of doing so), especially via unintended consequences. Questioning (skeptic) - Informed Consent.
Skin Cancer
 Skin Cancer are cancers that arise from the
Skin. They are due to the development of abnormal
cells that have the
ability to invade or spread to other parts of the body. More than 1
million cases of skin cancer are diagnosed in the United States each year.
Skin Cancer are cancers that arise from the
Skin. They are due to the development of abnormal
cells that have the
ability to invade or spread to other parts of the body. More than 1
million cases of skin cancer are diagnosed in the United States each year.There are 3 Main Types of Skin Cancers
1. Basal-Cell Skin Cancer is the most common skin cancer.
2. Squamous-Cell skin Cancer is cancer that begins from squamous cells, a type of skin cell.
3. Melanoma is a type of cancer that develops from the pigment-containing cells known as melanocytes, which are melanin-producing neural-crest derived cells located in the bottom layer (the stratum basale) of the skin's epidermis, the middle layer of the eye (the uvea), the inner ear, meninges, bones, and heart.
Melanin is the pigment primarily responsible for skin color. Once synthesised, melanin is contained in a special organelle called a melanosome and moved along arm-like structures called dendrites, so as to reach the keratinocytes.
Approximately 5.4 million basal and squamous cell skin cancers are diagnosed each year.
Carcinoma is a type of cancer that develops from epithelial cells. Specifically, a carcinoma is a cancer that begins in a tissue that lines the inner or outer surfaces of the body, and that generally arises from cells originating in the endodermal or ectodermal germ layer during embryogenesis. Carcinomas occur when the DNA of a cell is damaged or altered and the cell begins to grow uncontrollably and become malignant. It is from the Greek καρκίνωμα 'karkinoma' meaning sore, ulcer, or cancer, itself derived from karkinos 'crab.
Deep learning algorithm does as well as dermatologists in identifying skin cancer
USC Viterbi researchers invent waterproof patch to monitor UV ray exposure Color-changing smart material sensor will alert user that it’s time to get out of the sun.
Skin Cancer - Skin Cancer - Skin Cancer
Moles (images of Benign or Malignant) - Wart or a Mole?
Promising new drug stops spread of melanoma by 90 percent.
Some skin cancers may start in hair follicles. Some of the most deadly skin cancers may start in stem cells that lend color to hair, and originate in hair follicles rather than in skin layers. Grey Hair.
Scleroderma is a long term autoimmune disease that results in hardening of the skin. In the more severe form, it also affects internal organs. The cause is unknown. The underlying mechanism involves the body's immune system attacking healthy tissues. There is a strong association with certain mutations in HLA genes. Environmental factors have also been implicated.
Myocardin is a protein that in humans is encoded by the MYOCD gene. Myocardin is a smooth muscle and cardiac muscle-specific transcriptional coactivator of serum response factor. When expressed ectopically in nonmuscle cells, myocardin can induce smooth muscle differentiation by its association with serum response factor (SRF; MIM 600589).[supplied by OMIM].
Beneficial skin bacteria protect against skin cancer. A strain of S. epidermidis was shown to produce a molecule that kills cancer cells and inhibits the development of skin tumors on mice. UC San Diego Health. Staphylococcus epidermidis strain produces the chemical compound 6-N-hydroxyaminopurine, 6-HAP is a molecule that impairs the creation of DNA, known as DNA synthesis, and prevents the spread of transformed tumor cells as well as the potential to suppress development of UV-induced skin tumors.
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress.
Principles and Practice of Photoprotection. Summarizes the beneficial roles of photoprotection in relation to skin cancers, photoaging, photodermatoses, autoimmune and other skin conditions.
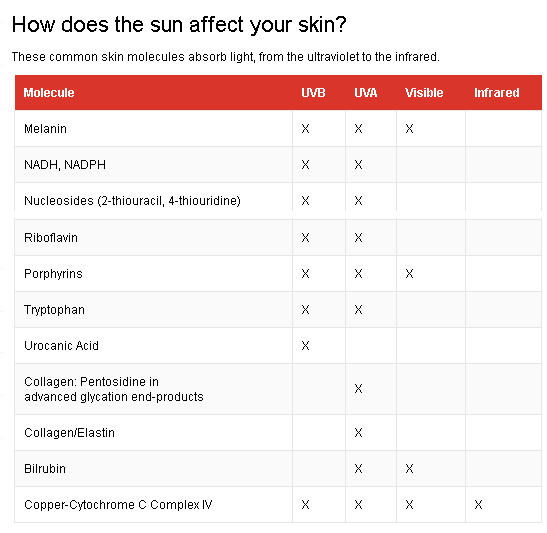 Sunburn is a form
of radiation burn that affects living tissue, such as skin, that results
from an overexposure to ultraviolet (UV) radiation, commonly from the
sun.
Common symptoms in humans and other animals include red or reddish skin
that is hot to the touch, pain, general fatigue, and mild dizziness.
Degrees of Burns. An excess of UV radiation can be life-threatening in extreme cases.
Excessive UV radiation is the leading cause of primarily non-malignant
skin tumors. Sunburn is an inflammatory response in the skin triggered by
direct DNA damage by UVR. When the skin cells' DNA is overly damaged by
UV Radiation, type I cell-death is
triggered and the skin is replaced. Sun protective measures including
sunscreen and sun protective clothing is widely accepted to prevent
sunburn and some types of skin cancer. Special populations including
children are especially susceptible to sunburn and protective measures
should be used. Skin Types: Type I: Pale
white skin, burns easily, does not tan. Type II: White skin, burns easily,
tans with difficulty. Type III: White skin, may burn but tans easily. Type
IV: Light brown/olive skin, hardly burns, tans easily. Type V: Brown skin,
usually does not burn, tans easily. Type VI: Black skin, very unlikely to
burn, becomes darker with UVR exposure. Lower the
Risk: Limit sun exposure between the hours of 10am and 4pm, when UV
rays are the strongest. Seek shade when UV rays are most intense. Do not burn.
Use
sunscreen. Wear sun-protective clothing including a wide brim
hat, sunglasses, and tightly-woven, loose-fitting clothing. Avoid tanning
beds and artificial UV exposure. Treatment:
For pain relief, take cool baths or showers frequently. Use soothing
moisturizers that contain aloe vera or soy. Hydrocortisone creams that can
be purchased over-the-counter can also be used on areas that are painful,
however avoid creams that end in "caine" as these can be more irritating.
Anti-inflammatory medications such as ibuprofen or aspirin can help with
pain. Keep hydrated and
drink extra water. If your sunburn Blisters, do not pop them. Instead, let
them heal on their own. Protect sunburned skin with loose clothing when
going outside to prevent further damage. The
UV Index indicates the risk of
getting a sunburn at a given time and location.
Contributing Factors Include: The time of day. In most locations,
the sun's rays are strongest between approximately 10am and 4pm daylight
saving time. Cloud cover. UV
is partially blocked by clouds; but even on an overcast day, a significant
percentage of the sun's damaging UV radiation can pass through clouds.
Proximity to Reflective Surfaces, such as
water, sand, concrete, snow, and ice. All of these reflect the sun's rays
and can cause sunburns. The
season of the year. The position of the sun in late spring and early
summer can cause a more-severe sunburn. Altitude.
At a higher altitude it is easier to become burnt, because there is less
of the earth's atmosphere to block the sunlight. UV exposure increases
about 4% for every 1000 ft (305 m) gain in elevation. Proximity to the
equator (latitude). Between the polar and tropical regions, the closer to
the equator, the more direct sunlight passes through the atmosphere over
the course of a year. For example, the southern United States gets fifty
percent more sunlight than the northern United States.
Sunburn is a form
of radiation burn that affects living tissue, such as skin, that results
from an overexposure to ultraviolet (UV) radiation, commonly from the
sun.
Common symptoms in humans and other animals include red or reddish skin
that is hot to the touch, pain, general fatigue, and mild dizziness.
Degrees of Burns. An excess of UV radiation can be life-threatening in extreme cases.
Excessive UV radiation is the leading cause of primarily non-malignant
skin tumors. Sunburn is an inflammatory response in the skin triggered by
direct DNA damage by UVR. When the skin cells' DNA is overly damaged by
UV Radiation, type I cell-death is
triggered and the skin is replaced. Sun protective measures including
sunscreen and sun protective clothing is widely accepted to prevent
sunburn and some types of skin cancer. Special populations including
children are especially susceptible to sunburn and protective measures
should be used. Skin Types: Type I: Pale
white skin, burns easily, does not tan. Type II: White skin, burns easily,
tans with difficulty. Type III: White skin, may burn but tans easily. Type
IV: Light brown/olive skin, hardly burns, tans easily. Type V: Brown skin,
usually does not burn, tans easily. Type VI: Black skin, very unlikely to
burn, becomes darker with UVR exposure. Lower the
Risk: Limit sun exposure between the hours of 10am and 4pm, when UV
rays are the strongest. Seek shade when UV rays are most intense. Do not burn.
Use
sunscreen. Wear sun-protective clothing including a wide brim
hat, sunglasses, and tightly-woven, loose-fitting clothing. Avoid tanning
beds and artificial UV exposure. Treatment:
For pain relief, take cool baths or showers frequently. Use soothing
moisturizers that contain aloe vera or soy. Hydrocortisone creams that can
be purchased over-the-counter can also be used on areas that are painful,
however avoid creams that end in "caine" as these can be more irritating.
Anti-inflammatory medications such as ibuprofen or aspirin can help with
pain. Keep hydrated and
drink extra water. If your sunburn Blisters, do not pop them. Instead, let
them heal on their own. Protect sunburned skin with loose clothing when
going outside to prevent further damage. The
UV Index indicates the risk of
getting a sunburn at a given time and location.
Contributing Factors Include: The time of day. In most locations,
the sun's rays are strongest between approximately 10am and 4pm daylight
saving time. Cloud cover. UV
is partially blocked by clouds; but even on an overcast day, a significant
percentage of the sun's damaging UV radiation can pass through clouds.
Proximity to Reflective Surfaces, such as
water, sand, concrete, snow, and ice. All of these reflect the sun's rays
and can cause sunburns. The
season of the year. The position of the sun in late spring and early
summer can cause a more-severe sunburn. Altitude.
At a higher altitude it is easier to become burnt, because there is less
of the earth's atmosphere to block the sunlight. UV exposure increases
about 4% for every 1000 ft (305 m) gain in elevation. Proximity to the
equator (latitude). Between the polar and tropical regions, the closer to
the equator, the more direct sunlight passes through the atmosphere over
the course of a year. For example, the southern United States gets fifty
percent more sunlight than the northern United States. Study gives insight into sun-induced DNA damage and cell repair. Ultraviolet light from the sun is a ubiquitous carcinogen that can inflict structural damage to the cellular DNA. As DNA carries important blueprints for cellular functions, failure in removing and restoring damaged parts of DNA in a timely fashion can have detrimental outcomes and lead to skin cancers in humans. Min and her team showed how the repair protein Rad4/XPC would bind to one such UV-induced DNA damage--6-4 photoproduct -- to mark the damaged site along the DNA in preparation for the rest of the nucleotide excision repair (NER) process in cells. Structure and mechanism of pyrimidine-pyrimidone (6-4) photoproduct recognition by the Rad4/XPC nucleotide excision repair complex.
Sunscreen is a lotion, spray, gel or other topical product that absorbs or reflects some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn. Diligent use of sunscreen can also slow or temporarily prevent the development of wrinkles, moles and sagging skin.
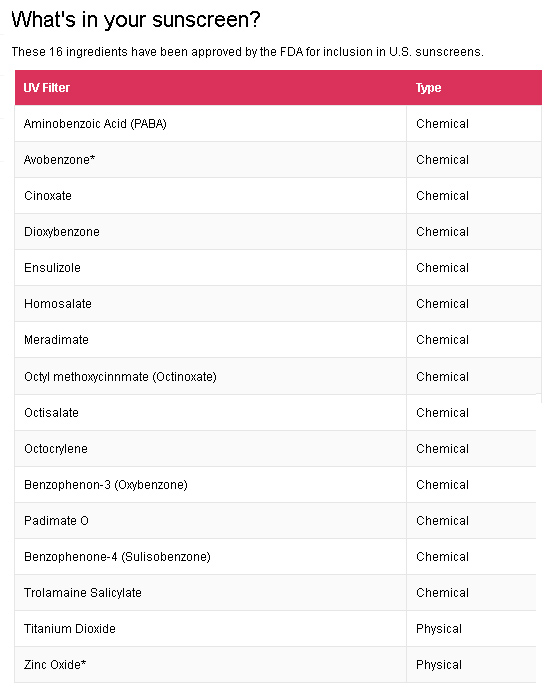 Oxybenzone is an
organic compound. It is a pale-yellow solid that is readily soluble in
most organic solvents. Oxybenzone belongs to the class of aromatic ketones
known as benzophenones. It is a component of many sunscreen lotions and is
used as an additive in plastics to protect them from UV degradation.
Benzophenones (along with three other active ingredients)[which?] in
sunscreens have been linked to coral bleaching. Around 3,500 sun
protection products containing oxybenzone are currently available to
consumers worldwide.
Oxybenzone is an
organic compound. It is a pale-yellow solid that is readily soluble in
most organic solvents. Oxybenzone belongs to the class of aromatic ketones
known as benzophenones. It is a component of many sunscreen lotions and is
used as an additive in plastics to protect them from UV degradation.
Benzophenones (along with three other active ingredients)[which?] in
sunscreens have been linked to coral bleaching. Around 3,500 sun
protection products containing oxybenzone are currently available to
consumers worldwide.
Coral Bleaching occurs when coral polyps expel algae that live inside their tissues, and as the algae provide the coral with up to 90% of its energy, after expelling the algae the coral begins to starve.
Octyl Methoxycinnamate is an organic compound that is an ingredient in some sunscreens and lip balms. It is an ester formed from methoxycinnamic acid and (RS)-2-ethylhexanol. It is a clear liquid that is insoluble in water.
Hippopotamus sweat blocks the sun's harmful rays and fights disease-causing microbes.
Skin Tag is a small benign tumor that forms primarily in areas where the skin forms creases, such as the neck, armpit and groin. They may also occur on the face, usually on the eyelids. Perianal skin tags can be associated with Crohn's disease. Acrochorda are generally harmless and painless and usually do not grow or change over time. Though tags up to a half-inch long have been seen, they are typically the size of a grain of rice. The surface of an acrochordon may be smooth or irregular in appearance and is often raised from the surface of the skin on a fleshy stalk called a peduncle. Microscopically, an acrochordon consists of a fibrovascular core, sometimes also with fat cells, covered by an unremarkable epidermis. However, tags may become irritated by shaving, clothing, jewellery or eczema. Skin hanger is a tiny, benign, outpouching of skin that is typically connected to the underlying skin by a thin stalk. Skin tags look like tiny bits of "hanging" skin and typically occur in sites where clothing rubs against the skin or where there is skin-to-skin friction, such as the underarms, neck, upper chest, and groin. Skin tags are not present at birth and their frequency increases with age. Skin tags can be observed in about 25% of adults. Studies have shown a genetic predisposition to the development of skin tags. Therefore, skin tags can run in families. A skin tag is medically termed an acrochordon. Sometimes, other terms have been used to refer to skin tags. These include soft warts (although they do not represent true warts), soft fibromas, fibroepithelial polyps (FEP), fibroma pendulans, and pedunculated fibroma.
A Sunburned Man Found A Lump On His Arm. This Is What Happened To His Brain (youtube) - metastatic melanoma.
Cancer in Pets
Not only do humans get abused by cancer and the cost of cancer treatments, but our pets do as well.
Dogs and Cancer - Cancer in Cats: Types, symptoms, prevention and treatments.
Cancer in Pets - Signs of Cancer in Pets - Pet Cancer
Pet Therapy